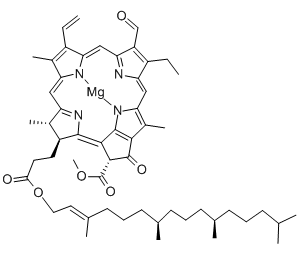
Structural formula
| Business number | 05CG |
|---|---|
| Molecular formula | C55H70MgN4O6 |
| Molecular weight | 907.47 |
| label |
None yet |
Numbering system
CAS number:519-62-0
MDL number:MFCD00079053
EINECS number:208-272-4
RTECS number:None
BRN number:4122778
PubChem ID:None
Physical property data
1. Character: light red hexagonal or quadrilateral plate crystal
2. Density (g/ cm3,25/4): Undetermined
3. Relative vapor density (g/cm3,AIR=1): Undetermined
4. Melting point (ºC):183-185
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,8kPa): Undetermined
7. Refractive Index: Undetermined
8. Flash Point (ºF): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of partition coefficient (water): Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:Soluble in methanol, ethanol and ethyl acetate, almost insoluble in ether and acetone
Toxicological data
None yet
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
None yet
Compute chemical data
1. Hydrophobic parameters Calculate the reference value (XlogP):
2. Hydrogen Bonding Number of donors: 0
3. Hydrogen Bonding Number of receptors: 10
4. Rotatable Number of chemical bonds: 23
5. Interchange Number of isomers: 3
6. Topological molecules Polar surface area (TPSA): 114
7. Heavy Atom Quantity: 66
8. Surface charge :0
9. Complexity :2180
10. Isotope atomic number:0
11. Determine the number of atomic stereocenters:2
12. Uncertain number of atomic stereocenters:3
13. Determine the number of stereocenters of chemical bonds:1
14. Uncertain number of chemical bond stereocenters:0
15. Number of covalent bond units:2
Properties and stability
Chlorophyllc: slightly red hexagon Or quadrilateral plate crystal. Soluble in methanol, ethanol and ethyl acetate, almost insoluble in ether and acetone. Maximum absorption value (acetone)628,580,442nm .
Storage method
Stored in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Most of them use plants (such as spinach, etc.) or dried silkworm sand as raw materials to extract chlorophyll. For example, the following method can be used to extract chlorophyll from silkworm sand. Organic solvent extraction: take clean silkworm sand and use 70%Make the above industrial ethanol into a slurry, filter out the dark green solution, and then dry it. Another operation is to 45Parts of petroleum ether,15 parts of methanol, 4mix benzene, mix with clean silkworm sand to make a slurry, filter, and wash the filtrate with water4times. Add a small amount of sodium sulfate to the organic extract to remove residual moisture. Filter and recover the solvent from the filtrate to obtain chlorophyll. Physical separation: 0.4mol/LDilute sucrose liquid and0.06mol/LPotassium phosphate dilute solution (pHfor6-7) Press1:1Mix into buffer medium. per2kgSilkworm Sand Plus1LMix the left and right buffer media evenly, filter out the green suspension with multiple layers of emery cloth, and centrifuge in a low-temperature centrifuge5-10min, wash the precipitate with water , centrifuge again to obtain chlorophyll precipitate. Chlorophyll is treated with oxalic acid to obtain magnesium-free pheophytin, and then magnesium is introduced and converted back into chlorophyll. Removal of phytol and magnesium with strong acid results in pheophorbide, and phytol can also be reintroduced. Hydrolysis removes phytol and methanol to obtain chlorophyllin. Most of them use plants (such as spinach, etc.) or dried silkworm sand as raw materials to extract chlorophyll. For example, the following method can be used to extract chlorophyll from silkworm sand. Organic solvent extraction: take clean silkworm sand and use 70%Make the above industrial ethanol into a slurry, filter out the dark green solution, and then dry it. Another operation is to 45Parts of petroleum ether,15 parts of methanol, 4parts of benzene mixed with Mix clean silkworm sand into pulp, filter, and wash the filtrate with water4times. Add a small amount of sodium sulfate to the organic extract to remove residual moisture, filter, and recover the solvent from the filtrate to obtain chlorophyll. Physical separation: 0.4mol/LDilute sucrose solution and 0.06mol/LPotassium Phosphate Solution (pH for6-7) Press 1:1 Mix into buffered medium. Every2kgSilkworm sand plus 1LLeft and right buffer media, Mix evenly, filter out the green suspension with multi-layer emery cloth, and centrifuge in a low-temperature centrifuge5-10min, wash the precipitate with water, and centrifuge again to obtain the chlorophyll precipitate. Chlorophyll is treated with oxalic acid to obtain magnesium-free pheophytin, and then magnesium is introduced and converted back into chlorophyll. Removal of phytol and magnesium with strong acid results in pheophorbide, and phytol can also be reintroduced. Hydrolysis removes phytol and methanol to obtain chlorophyllin.
Purpose
Chlorophyll is used in soaps , mineral oil, wax and essential oil coloring. Derivatives of chlorophyll or chlorophyllin, such as copper chlorophyll[11006-34-1], sodium iron chlorophyllate, sodium copper chlorophyllin, used as colorants and deodorants in food, candy, beverages, toothpaste, etc. Chlorophyllin derivatives can be used together with the fungicides benzalkonium chloride, halocarban, etc. to formulate smelly cosmetics.

 微信扫一扫打赏
微信扫一扫打赏

