Background and overview[1][2]
Phenylsulfonyl acetonitrile can be used as an intermediate for pharmaceutical and chemical synthesis. If benzenesulfonyl acetonitrile is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eyes come into contact, separate Rinse the eyelids with running water or saline and seek medical attention immediately. If ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately.
Apply [1-5]
Phenylsulfonyl acetonitrile can be used as an intermediate for pharmaceutical and chemical synthesis. Examples of its application are as follows:
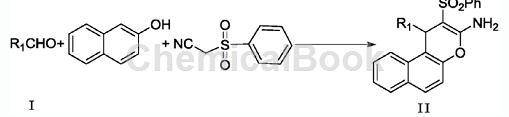
1. In the process of preparing naphthopyran derivatives using ionic liquid catalyzed one-pot method, the method is carried out as follows: aldehyde derivatives shown in formula I, β-naphthol, benzene Sulfonyl acetonitrile and ionic liquid catalyst are added to the solvent, and stirred at 0-80°C and normal pressure for 2-18 hours. After the reaction is complete, the reaction liquid is obtained and after post-processing, 2-amino-3-benzene represented by formula II is obtained. Sulfone-4H-naphthopyran derivatives. The present invention prepares 2-amino-3-phenylsulfone-4H-naphthopyran derivatives with mild reaction conditions, short reaction time, convenient separation and purification, good yield, and easy expansion of substituents; and using ionic liquid catalysis, It has the advantages of being pollution-free, environmentally friendly, and the catalyst can be reused, making it a promising synthesis method.
2. Used to catalyze the preparation of 2-amino-3-benzenesulfonyl-4H-pyran derivatives. In this preparation reaction, the molar ratio of aromatic aldehyde, 5,5-dimethyl-1,3-cyclohexanedione and benzenesulfonyl acetonitrile is 1:1:1. The molar amount of the alkaline ionic liquid catalyst is the aromatic aldehyde used. 8~10%, the volume of the reaction solvent 95% methanol aqueous solution in milliliters is 5~8 times the amount of the aromatic aldehyde in millimoles, the reflux reaction time is 2~5h, cool to room temperature after the reaction is completed, After suction filtration, the filter residue was washed with methanol and dried under vacuum to obtain 2-amino-3-benzenesulfonyl-4H-pyran derivatives. Compared with the preparation method of other alkaline ionic liquid catalysts, it has the characteristics of less catalyst usage, high raw material utilization rate and simple and convenient operation of the entire preparation process, which is convenient for large-scale industrial application. In addition, there are studies using polystyrene resin supported alkaline ionic liquid as a catalyst, and the molar ratio of aromatic aldehyde, 5,5-dimethyl-1,3-cyclohexanedione and phenylsulfonyl acetonitrile in the preparation reaction is 1 (1~1.2): 1. The molar weight of the catalyst is 10-16% of the molar weight of aromatic aldehyde. The volume of ethanol aqueous solution as the reaction solvent is 5-8 times the molar weight of aromatic aldehyde. The reflux reaction is 2.0-4.2h. The reaction After completion, perform suction filtration while hot, cool the filtrate to room temperature, let it stand, and perform suction filtration. The filter residue is washed with ethanol aqueous solution and dried under vacuum to obtain 2-amino-3-benzenesulfonyl-4H-pyran derivatives. The above technical solution has the characteristics of low catalyst loss, high recycling times, simple and convenient operation of the entire preparation process, and a high degree of greenness, and is convenient for large-scale industrial application.
3. Preparation of 2-keto-3-phenylsulfonyl-6-ethylene glycol ketal-5,6,7,8-tetrahydroquinoline, the reaction formula is as follows:
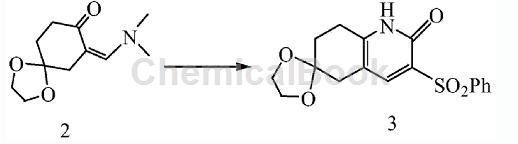
The specific preparation process is: In a 2L three-necked flask, add compound 2 (211g, 1mol) and phenylsulfonylacetonitrile (181g, 1mol) obtained in the previous step respectively. The chemical structural formula of phenylsulfonylacetonitrile is: C 6H5SO2CH2CN. Then add 500L of ethanol and install a reflux condenser to heat the reflux reaction. TLC detected the reaction progress. After 1 hour of reaction, the reaction was complete. Stop heating and cool naturally. At this time, a large amount of solid precipitated. Filter the solid, wash with a small amount of ethanol, and dry to obtain a white solid 2-keto-3-phenylsulfonyl-6-ethylene glycol ketal-5,6,7,8-tetrahydroquinoline (compound 3 ) 185g (yield 53.3%).
4. Prepare 2-methoxy-7,8-dihydroquinolin-6(5H)-one. The synthesis method is:
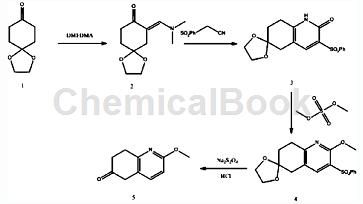
The synthesis method includes the following steps: a. Compound 1 and DMADMF (N,N-dimethylformamide dimethyl acetal) are added to the DMF (N,N-dimethylformamide) solution, and the temperature is raised to The reaction was carried out with constant stirring at 70°C for 2 hours; then DMF (N,N-dimethylformamide) and remaining DMADMF (N,N-dimethylformamide dimethyl acetal) were removed under reduced pressure to obtain compound 2 ; b. Add compound 2 and phenylsulfonyl acetonitrile in step a to ethanol, and heat to reflux for 1 hour at 85°C, then filter the obtained product, wash the obtained solid with ethanol, and then dry it That is, compound 3 is obtained; c. Add dimethyl sulfate dropwise to the reaction system consisting of compound 3, potassium carbonate and tetrahydrofuran in step b, and reflux and heat for 2 hours at 80°C, cool to room temperature, and then After three extractions with ethyl acetate, combine the organic phases, concentrate to dryness, and finally beat with a small amount of ethanol, filter and dry to obtain compound 4; d. Add DMF (N, N-dimethylmethane) to compound 4 in step c. formamide) and water, and then add sodium dithionite in batches, and then react at 80°C for 5 hours; then cool to room temperature; then filter to collect the filter cake, and then add concentrated hydrochloric acid to the filter cake , stir and heat to reflux for 2 hours, adjust the pH with saturated sodium carbonate solution, then extract three times with ethyl acetate, combine the organic phases, and concentrateto dryness, and then beat with ethanol to obtain compound 5, that is, 2-methoxy-7, 8-dihydroquinolin-6(5H)-one.
Main reference materials
[1] CN201710129677.9 A 2-amino-3-benzenesulfonyl-4H-pyran derivative and its preparation method and catalyst for preparation
[2] CN201810534353.8 A method for preparing naphthopyran derivatives using an ionic liquid catalyzed one-pot method
[3] CN201610082755.X A method for catalytically preparing 2-amino-3-benzenesulfonyl-4H-pyran derivatives
[4] CN201410579839.5 A synthesis method of 2-methoxy-6-one-5,6,7,8-tetrahydroquinoline
[5] CN201710953791.3 A synthesis method of 2-methoxy-7,8-dihydroquinoline-6(5H)-one

 微信扫一扫打赏
微信扫一扫打赏

