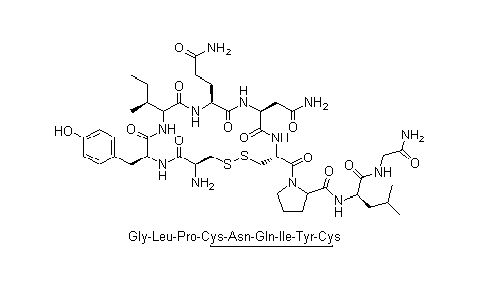
Structural formula
| Business number | 013X |
|---|---|
| Molecular formula | C43H66N12O12S2 |
| Molecular weight | 1007.2 |
| label |
N-Benzyloxycarbonyl-S-benzyl cysteinyl tyrosyl isoleucyl glutaminyl asparaginyl (S-benzyl) cysteinyl prolyl, oxytocin, A-Hypophamine, Oxytocic hormone, oxytocin |
Numbering system
CAS number:50-56-6
MDL number:MFCD00076731
EINECS number:200-048-4
RTECS number:RS7534000
BRN number:3586108
PubChem number:24897975
Physical property data
1. Properties: White amorphous powder with slight odor 2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 192-194°C
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC , 5.2 kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º): -26.2°, C=0.53, DIOXANE
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25 ºC ): Undetermined
12. Saturated vapor pressure (kPa, 60 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, soluble in acetone, butanol and dilute acetic acid, insoluble in diethyl ether and petroleum ether
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -2.6
2. Number of hydrogen bond donors: 12
3. Number of hydrogen bond acceptors: 13
p>
4. Number of rotatable chemical bonds: 17
5. Number of tautomers: 1001
6. Topological molecular polar surface area (TPSA): 400
7. Number of heavy atoms: 69
8. Surface charge: 0
9. ���Hurility: 1870
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 9
12. Uncertain atomic stereocenter Number of stereocenters of chemical bonds: 0
13. Number of stereocenters of determined chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Total Number of price key units: 1
Properties and stability
None
Storage method
Brown glass bottle with light-sealed packaging. Store in low temperature and dry place.
Synthesis method
1. Both oxytocin and vasopressin are nonapeptides. There is a basic amino acid residue in the vasopressin molecule, and their isoelectric points are different. The isoelectric point of oxytocin is 7.7 and that of vasopressin is 10.9, which means that vasopressin is highly alkaline. When the extract containing these two hormones is passed into an artificial zeolite column, vasopressin easily forms positively charged ions and most of them are adsorbed, while oxytocin flows out through the zeolite column. The effluent oxytocin solution can be treated with bentonite to obtain purer oxytocin.
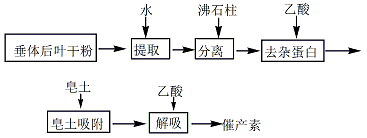
Extraction of posterior pituitary gland dry powder ↓water separation ↓zeolite column to remove impurity protein ↓acetic acid
Bentonite adsorption ↓bentonite desorption ↓oxytocin acetate
The pituitary gland Add 100g of dry leaf powder and about 30g of quartz powder into 1.4L of distilled water, grind in a ball mill and extract for 45 minutes, centrifuge, and collect the supernatant. Add 1.4L, 1.3L, and 1.3L of water to the residue and extract 3 more times according to the above method. Combine the supernatants from 4 times of centrifugation. Add 1500g of the treated artificial zeolite to 20L of 0.25% acetic acid solution, stir and put it into the exchange column. When the 0.25% acetic acid solution in the exchange column drops to 2 to 3cm below the surface of the zeolite, immediately add the extraction solution and adjust the flow rate appropriately. , collect the white turbid liquid that begins to flow out of the adsorption column. When the liquid level of the extraction liquid drops to the surface of the zeolite, add distilled water immediately, and stop collecting when the turbid liquid flows out. The extract solution flowing through the zeolite was adjusted to pH=3.5 with glacial acetic acid, then rapidly heated to 95°C in a water bath for 3 minutes, cooled immediately, and placed in a cold room at 0 to 5°C overnight. Filter the next day to get a clear filtrate. Then, add the previously treated 10% bentonite slurry (usually 3ml per 100ml) to the filtrate while stirring, and stir continuously for 1 hour. The supernatant should be checked for complete adsorption (check with 20% sulfosalicylic acid solution, it should be No precipitation occurs), if the adsorption is incomplete, additional bentonite slurry should be added for adsorption. The 2 bentonite precipitations merged. The bentonite was then desorbed with 1% acetic acid solution. The total dosage is 5L and desorbed in 4 times. The dosages are 1.6L, 1.4L, 1.2L and 0.8L respectively. The 4 desorption liquids are combined. Determine the content of oxytocin and vasopressin in the desorption solution, and calculate the oxytocin potency. If the vasopressin does not exceed the limit, the oxytocin solution is obtained.
Purpose
1. It has a stimulating effect on smooth muscles and can cause the contraction of uterine smooth muscles, so it has an oxytocin effect. It can be used to induce labor, induce labor and uterine bleeding caused by weak uterine contractions after abortion. Intranasal instillation can promote milk excretion. It also has a slight hemostatic effect.
extended-reading:https://www.bdmaee.net/nn-dimethyl-ethanolamine/extended-reading:https://www.cyclohexylamine.net/dabco-t-12-tin-catalyst-dabco-t-12-catalyst-t-12/extended-reading:https://www.bdmaee.net/nt-cat-la-300-catalyst-cas10861-07-1-newtopchem/extended-reading:https://www.morpholine.org/category/morpholine/page/2/extended-reading:https://www.newtopchem.com/archives/44519extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/137-3.jpgextended-reading:https://www.bdmaee.net/pc-cat-np40-catalyst-trisdimethylaminopropylhexahydrotriazine/extended-reading:https://www.newtopchem.com/archives/954extended-reading:https://www.bdmaee.net/butyl-tin-triisooctoate-cas23850-94-4-butyltin-tris/extended-reading:https://www.bdmaee.net/reactive-composite-catalyst/

 微信扫一扫打赏
微信扫一扫打赏

