Background and overview[1][2]
Methylaminoacetonitrile hydrochloride can be used as an intermediate for pharmaceutical and chemical synthesis. In the prior art, the main methods for synthesizing aminoacetonitrile are as follows: Sodium cyanide method: using amine, sodium cyanide, and formaldehyde in the presence of magnesium salts such as magnesium chloride or magnesium sulfate; liquid hydrocyanic acid method: using Amine, formaldehyde, and liquid hydrocyanic acid are used as raw materials, and formaldehyde and hydrocyanic acid are added dropwise at the same time. After reaction, extraction and crystallization, aminoacetonitrile is obtained, with a yield of 80 to 95% and a content of 92 to 98%; hydroxyacetonitrile method: using hydrocyanic acid It first reacts with formaldehyde to form hydroxyacetonitrile, and then condenses with amine to obtain aminoacetonitrile.
Among the above methods, although the liquid hydrocyanic acid method has a higher yield, the operation is risky. In order to obtain liquid hydrocyanic acid, a refrigeration system is required, which requires low equipment investment, high cost, and is uneconomical; while hydroxyacetonitrile The reaction step of the method is long, the operation is complicated, the equipment investment is large, and the cost is high; the sodium cyanide method has the advantage of simple operation, but the yield is low, only about 50%.
Application
Methylaminoacetonitrile hydrochloride can be used as an intermediate for pharmaceutical and chemical synthesis. Examples of its applications are as follows:
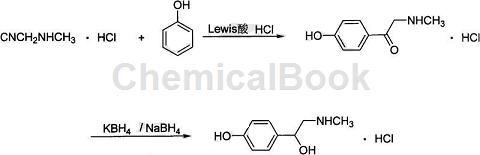
1. Used to prepare synephrine (2-methylamino-1-(4-hydroxyphenyl)ethanol) hydrochloride.
Synephrine is an adrenergic α-receptor agonist that can constrict blood vessels and increase blood pressure. It is widely used in health care industries such as medicine, food, and beverages. It also has the effects of increasing metabolism, increasing calorie consumption, increasing energy levels, oxidizing fat, and losing weight. At present, the preparation of synephrin is mainly extracted from the traditional Chinese medicine Citrus aurantium, and there are very few reports on chemical synthesis methods.
Some research provides a preparation method that uses methylaminoacetonitrile hydrochloride as raw material, reacts with phenol and HCl under the catalysis of Lewis acid to generate 2-methylamino-1-(4-hydroxyphenyl). Ethyl ketone hydrochloride is then reduced by sodium borohydride or potassium borohydride to obtain 2-methylamino-1-(4-hydroxyphenyl)ethanol hydrochloride. The product purity can reach more than 99%, the total yield is 67.5%, the process is simple to operate, and it is easy to realize large-scale production.
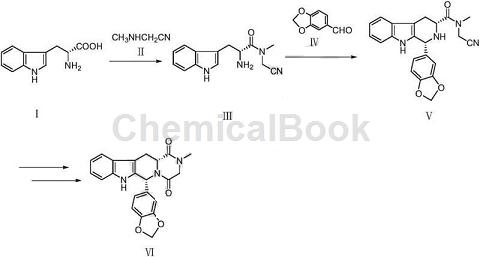
2. Prepare Tadalafil.
Tadalafil is a phosphodiesterase type V (PDE5) inhibitor. When sexual stimulation causes the local release of nitric oxide, PDE5 is inhibited by tadalafil, which increases the level of cyclic guanosine monophosphate in the corpus cavernosum of the penis. , which causes the smooth muscles to relax and blood to flow into the penile tissue, producing an erection. The compound was originally developed by GlaxoSmithKline and subsequently transferred to ICOS, and then jointly developed by ICOS and Eli Lilly. Approved by the FDA in 2003, tadalafil was marketed in the United States as a drug for the treatment of male erectile dysfunction.
In early 2014, the drug was approved by the US FDA as a new indication for the treatment of benign prostatic hyperplasia, and the market capacity will be broader in the future. Some studies have developed a preparation process. 1) D-tryptophan and methylaminoacetonitrile or methylaminoacetonitrile hydrochloride are used as starting raw materials to condense to obtain the compound represented by formula III. 2) Formula III and piperonal undergo Pictet- After Spengler cyclization reaction, the compound represented by Formula V is crystallized. 3) Compound V is hydrolyzed under acidic or alkaline conditions and then condensed to obtain the target product Tadalafil. The above method avoids the use of chemical raw materials such as sulfoxide dichloride, chloroacetyl chloride, and methylamine, which are highly toxic, cause serious environmental pollution, and are flammable and explosive. The reaction method is simple, the operation is convenient, and the yield is high.
Preparation [1]
A method for preparing methylaminoacetonitrile hydrochloride, including: (1) using methylamine hydrochloride, sodium cyanide and formaldehyde as reaction raw materials, reacting in the presence of a catalyst to generate methylaminoacetonitrile; (2) The methylaminoacetonitrile reacts with hydrochloric acid to generate the methylaminoacetonitrile hydrochloride. In step (1), the reaction uses 3-mercaptopropionic acid as a catalyst and is carried out below 0°C. The catalyst dosage is 0.9 to 1.1 of sodium cyanide. times.
Main reference materials
[1] CN200810136271.4 A preparation method of methylaminoacetonitrile hydrochloride
[2] CN201010266417.4 A chemical synthesis method of synephrine hydrochloride
[3] CN201510502098.5 A synthesis method of tadalafil

 微信扫一扫打赏
微信扫一扫打赏

