Background and overview[1]
3-Acetonitrilecyclobutylamine hydrochloride can be used as a pharmaceutical synthesis intermediate.
Preparation[1-2]
Method 1: 3-acetonitrilecyclobutylamine hydrochloride is prepared as follows:
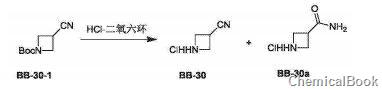
Compound BB-30-1 (0.200g, 1.10mmol) was dissolved in dichloromethane (2mL), and then hydrogen chloride dioxane solution (1mL, 4M) was added. The reaction solution was stirred at 20°C for 2 hours. The reaction solution was concentrated to obtain target compounds BB-30 and BB-30a (white solid, 0.130 g, yield: 99%), which were directly used in the next step of synthesis without isolation. MS(ESI) m/z: 83[M+H]+. MS(ESI)m/z: 101[M+H]+.
Method 2: 3-acetonitrilecyclobutylamine hydrochloride is prepared as follows:
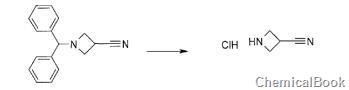
To a 50 mL flask, add 1-diphenylmethylazetidine-3-carbonitrile (500 mg, 2.01 mmol) and 1,2-dichloroethane (8.9 mL). 1-Chloroethyl chloroformate (285 μL, 2.61 mmol) was added and the reaction mixture was stirred at 70°C for 1.5 hours. After cooling to room temperature, methanol (8.9 mL) was added and the reaction mixture was stirred at 70°C for 1.5 hours. The reaction mixture was concentrated to dryness. The crude mixture was triturated in pentane to give a black solid (250 mg, quantitative).
Apply[1]
3-Acetonitrilecyclobutylamine hydrochloride can be used to prepare tert-butyl 3-cyanoazetidine-1-carboxylate:

To a solution of azetidine-3-carbonitrile hydrochloride (250mg, 2.01mmo) and triethylamine (1.12mL, 8.04mmol) in dichloromethane (10.2mL) at 5°C. Di-tert-butyl dicarbonate (482 mg, 2.21 mmol) was added in one portion. After stirring at room temperature for 16 hours, the reaction mixture was washed with 0.5 N HCl and brine and dried over sodium sulfate. The solvent was evaporated under reduced pressure and the crude mixture was purified by column chromatography (eluent: pentane/EtOAc 95/5 to 4/1) to give the title compound (190 mg, 52%) as a colorless oil. 1HNMR (CDCl3, 300MHz): δ (ppm): 4.29-4.05 (m, 4H), 3.48-3.29 (m, 1H), 1.44 (s, 9H).
Main reference materials
[1] CN102656158 – New heterocyclopropylamide compounds and their use as drugs
[2] CN201610070866.9 Benzofuran analogues as NS4B inhibitors

 微信扫一扫打赏
微信扫一扫打赏

