Background and overview[1]
Tetrakis (acetonitrile) palladium (II) bis (trifluoromethanesulfonic acid) can be used as an intermediate catalyst for pharmaceutical synthesis. If tetrakis(acetonitrile)palladium(II)bis(trifluoromethanesulfonic acid) is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water. If you feel discomfort, seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Application
Tetrakis (acetonitrile) palladium (II) bis (trifluoromethanesulfonic acid) can be used as an intermediate catalyst for pharmaceutical synthesis. Such as catalytic oxidation of alkynes to prepare 1,2-diones. One of the oxidation products of alkynes is 1,2-diketone, which is a very important class of organic compounds. It is included in a variety of physiologically active natural products and is also widely used in the synthesis of other heterocycles. compound. At present, the commonly used oxidants for the oxidation of alkynes mainly include the following types: high-priced metal oxidants (such as potassium permanganate, potassium dichromate), DMSO, osmium tetroxide, peroxides (such as hydrogen peroxide, ozone), etc. When using the above oxidants to catalytically oxidize alkynes to prepare 1,2-diketones, the reaction conditions are harsh, the selectivity is low, and the substrate range is narrow. The specific method is: using alkyne R1-C≡C-R2 as the reactant, using one of palladium chloride, palladium acetate, palladium bromide, palladium trifluoroacetate or palladium trifluoromethanesulfonate as the catalyst, and using copper The salt is used as a cocatalyst, and the mixed solution of ether and water is used as the solvent. The reaction is carried out at 40 to 100°C for 12 to 36 hours to prepare 1,2-diketone.
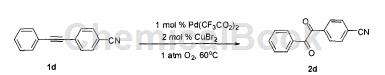
More specifically: put palladium trifluoroacetate (0.01mmol, 3.3mg), copper bromide (0.02mmol, 4.4mg), compound 1d (1.0mmol, 207mg), and tetrahydrofuran (1.0mL) into the reaction bottle in sequence. and water (1.0 mL). The system was connected to an oxygen bag, and then evacuated cyclically and replaced with oxygen three times. The system was then heated in an oil bath at 60°C for about 24 hours to obtain a crude product; it was cooled to room temperature and extracted with ethyl acetate (4.0 mL × 3). , the oxidation product 2d can be obtained through simple column chromatography, with a yield of 86%.
Preparation
Mix metal palladium oxide and deionized water, add an aqueous solution of trifluoromethanesulfonic acid dropwise at room temperature, 80° for one hour, cool down, filter, rinse, and concentrate the filtrate to obtain a solid, vacuum dry at 50°C, and activate at 260°C Tetrakis(acetonitrile)palladium(II)bis(triflate) was prepared.
Main reference materials
[1] CN200810021756.9 A method for catalytically oxidizing alkynes to prepare 1,2-diones

 微信扫一扫打赏
微信扫一扫打赏

