Background and overview[1]
Methoxyethyl acetoacetate can be used as a pharmaceutical synthesis intermediate.
Apply[1]
Methoxyethyl acetoacetate can be used to prepare cilnidipine intermediate 2-(3-nitrobenzylidene)methoxyethyl acetoacetate. Cilnidipine was developed by Fujifilm Corporation of Japan and was first launched in Japan in December 1995. The chemical name of cilnidipine is: racemic 2,6-dimethyl-4(3-nitrobenzene)-1,4-dihydropyridine-3,5-dicarboxylic acid-2-methoxyethyl (E )‑3‑phenyl‑2‑propenyl diester. Cilnidipine is a lipophilic dihydropyridine calcium antagonist that can bind to the dihydropyridine site of the L-type calcium channel on the vascular smooth muscle cell membrane, inhibiting the transmembrane influx of Ca2+ through the L-type calcium channel, thereby relaxing and expanding Vascular smooth muscle, which plays a role in lowering blood pressure. The specific steps are as follows:
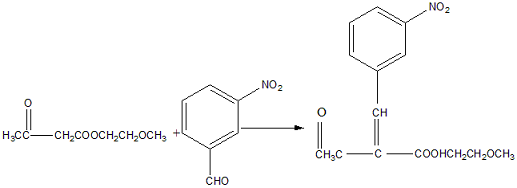
(1) Weigh 200g of methoxyethyl acetoacetate, add it to a 500ml three-necked flask, stir, cool down to about 5℃, add 25ml of concentrated sulfuric acid dropwise to ensure that the temperature is below 10℃, complete the dropwise addition in 40 minutes, After completion, add 100ml of ethyl acetate, and then add 151g of m-nitrobenzaldehyde in three equal parts; (2) React at room temperature for 2.5 hours, stop stirring, and leave overnight; (3) Add 450ml of ethyl acetate the next day to dissolve all the solids. Add 200 ml of purified water for extraction twice, and collect the organic layer; (4) Evaporate the ethyl acetate under reduced pressure, cool it with ice water, and crystallize; (5) Filter the obtained crystals and wash them with a small amount of ethanol to obtain a light yellow solid, 70 Dried at ℃, 208g of solid was obtained, with a yield of 71% and a content of 99.2% (high performance liquid chromatography analysis). Compared with the existing preparation method of cilnidipine condensation intermediate, the purity of the obtained target product is high, reaching more than 99%, and the yield is high, reaching more than 70%. Moreover, the reaction conditions are mild and the operation is simple.
Main reference materials
[1] Synthesis method of CN201110433851.12-(3-nitrobenzylidene)methoxyethyl acetoacetate

 微信扫一扫打赏
微信扫一扫打赏

