Overview【1】
Copper hydroxide is an important industrial material that can be used in medicine, mordants, pigments, feed additives, pesticides, paper dyes and catalysts. In recent years, it has also been found to have great potential in energy storage and sensors. .
Physical and chemical properties【1】
Copper hydroxide is a layered material of the orthorhombic crystal system. The space group is Cmc21. The crystal form is lepidocrocite γ-FeOOH type. Each unit cell has four copper hydroxide units. It is a ferroelectric substance that also has superconducting properties. Copper hydroxide is an important industrial material that can be used in medicine, mordants, pigments, feed additives, pesticides, paper dyes and catalysts. In recent years, it has also been found to have great potential in energy storage and sensors.
Preparation method【1】【2】
1. Preparation method of nano-Cu(OH)2
(1) Wet chemical method
The generalized wet chemical method refers to the general term for methods of preparing materials through chemical reactions involving the participation of a liquid phase. Commonly used methods include precipitation, sol-gel, and hydrothermal methods. This method is characterized by mild reaction conditions, low cost, simple operation, easy control of the morphology, composition and structure of the product, and wide applicability.
① Quickly add NaOH solution to the mixed aqueous solution of CuSO4?5H2O and H2O2 (auxiliary agent) at room temperature, stir for 15 minutes, and centrifuge to wash to obtain Cu(OH)2 nanowires. The study found that the morphology of Cu(OH)2 nanowires can be adjusted by the concentration of H2O2. High concentrations of H2O2 can produce longer Cu(OH)2 nanowires. At the same time, H2O2 can also promote the recombination of Cu(OH)2. Crystallization and directional growth.
② Use Cu(NO3)2 (0.2mol?L-1) and NaOH (0.2mol?L-1) as raw materials, mix them in a Y-type tubular reactor, react to produce a precipitate, and then place it in Stir in 0.2mol?L-1NaOH solution at a certain temperature (5, 20, 30, 40°C) for 1 hour, centrifuge, wash, and vacuum dry to obtain the final product. The research results show that: at a temperature of 5 and 20°C, the product is Cu(OH)2 nanowires; at 30°C, it is a Cu(OH)2/CuO mixture; at 40°C, the product is completely converted into CuO, and the product size changes with decreases as the temperature decreases.
③ Dissolve CuSO4?5H2O in distilled water, quickly add a certain amount of ammonia water under stirring conditions, and add NaOH solution drop by drop while stirring for 15 minutes. At this time, a blue precipitate will form. After continuing to stir for 15 minutes, wash the precipitate. Filter and dry at 35°C for 24h to obtain the final product. The study found that the product was Cu(OH)2 nanowires with a diameter of about 8 nm and a length of several hundred microns. The study also found that if the concentration of SO42- is too low, it is easy to form Cu(OH)2 nanoparticles; if the concentration of NH3 is too high, it is easy to form Cu(OH)2 nanoparticles; when the pH value is greater than 8, only irregular Cu(OH)2 nanoparticles are obtained. line, and if the NaOH concentration is higher than 4mol?L-1, only Cu(OH)2 nanoparticles can be obtained.
(2) Precursor method
The precursor method is a method that first prepares a complex precursor containing the target product element through a corresponding reaction, and then processes it with appropriate physical and chemical methods to obtain the target product. The precursor method has a wide range of applications, especially in the preparation of inorganic materials, such as metal oxide nanomaterials (CuO, SnO2, ZnO2, CdO, etc.), rare earth inorganic materials, etc.
① Using CuSO4 and urea as raw materials, the precursor Cu4SO4(OH)6 was prepared by hydrothermal method, and then the Cu4SO4(OH)6 was treated with NaOH strong alkali solution to obtain fishbone-shaped nano-Cu(OH)2.
② Add excess ammonia dropwise to CuSO4, then add an appropriate amount of Ba(OH)2 to precipitate SO42-, then centrifuge to obtain the tetraammine copper (II) hydroxide precursor solution, and heat it to reflux to lower the pH value. To 8~9, Cu(OH)2 precipitates out in the solution. The precipitate was washed several times with ammonia and ethanol, filtered and dried to obtain blue copper hydroxide powder.
③ Use CuCl2?2H2O, urea, and sodium 1-octane sulfonate (molar ratio 1:2:2) as raw materials, deionized water as solvent, stir for 1 hour, transfer the mixture to a glass bottle, and place in the oven at 373K Incubate at room temperature for 48h to obtain spherical Cu(OH)2Cl nanosheets, which are then placed in 1mol?L-1NaOH and stirred at room temperature for 1h to obtain spherical Cu(OH)2 formed by nanowire aggregation.
④ Use Cu(CH3COO)2?H2O as the raw material, ethanol as the solvent, then add diethyl oxalate, stir for 15 minutes, transfer to the reactor, react at 100°C for 12 hours to obtain the precursor CuC2O4?H2O; then condition at room temperature Stir and disperse CuC2O4?H2O into distilled water, then add NaOH solution drop by drop, continue stirring for 1 hour, filter and wash to obtain dandelion-shaped nano-Cu(OH)2. The reaction formula is as follows:
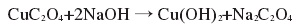
Figure 1 is the reaction formula for synthesizing copper hydroxide using the precursor method
(3) Template method
The template method can design a template in advance according to the size and morphology of the synthetic material. Based on the spatial confinement of the template and the regulation of the template agent, the size, morphology, structure, arrangement, etc. of the synthetic material can be controlled, regardless of It is a chemical reaction that occurs in the liquid phase or the gas phase, and the reactions are carried out in an effectively controlled area.
① Use Cu2(C11H23CO2)4?2H2O and Cu(C12H25SO4)2?4H2O as sheet-shaped nanoreactors, stir and disperse them in distilled water at room temperature, add NaOH under vigorous stirring, and Cu( OH)2 brown precipitate.
② Using CuCl2?2H2O as the raw material and CH2Cl2 containing 5 times the equivalent of linear butylamine as the solvent, synthesize the blue copper amine complex [CuX2(CnH2n+1NH2)2], and then add distilled water to form water-in-oil (H2O /CH2Cl2) system, blue floc immediately appeared in the CH2Cl2 organic phase.Generate drug resistance. Keshade 101 can be mixed with most pesticides (except for strongly alkaline pesticides).
Application:
1. Prevent and control tomato early blight: Before the onset or in the early stage of the onset, spray 135-200 grams of 77% wettable powder with water per acre.
2. To prevent anthracnose, late blight, powdery mildew, downy mildew, angular spot, stripe spot, etc. on vegetables and melon crops: spray with 500-700 times of 77% wettable powder.
3. Prevention and treatment of cucumber bacterial angular spot, tomato bacterial spot, watermelon leaf blight, cowpea angular spot, cowpea bacterial blight, lettuce soft rot, cabbage black rot, and ginger blast: incidence In the initial stage, spray 77% wettable powder 500-800 times, spray once every 7-10 days, and control 2-3 times in total.
4. Prevention and treatment of tomato and eggplant bacterial wilt: In the early stage of the disease, use 77% wettable powder 500 times to irrigate the roots. Each plant should be irrigated with 0.3~0.5 liters of the correct solution. Irrigate once every 10 days for a total of 3 times. ~4 times.
Main reference materials
[1]Xu Shuang. Research on the preparation and application performance of nanometer copper hydroxide for fungicides [D]. University of Chinese Academy of Sciences (Institute of Process Engineering, Chinese Academy of Sciences), 2018.
[2] Lu Linglan, Ma Jinming, Cui Yue, Ma Ying, Mu Cheng, Zhang Xiaoping, Zu Yunjiao. Preparation of stable copper hydroxide [J]. Tianjin Chemical Industry, 2007(03):40-41.
[3] Liu Xifang, Wu Xueqin. New copper hydroxide fungicide-Keshade[J]. Henan Agriculture, 1993(06):14.
[4] Broad-spectrum protective fungicide copper hydroxide (killable) [J]. Jilin Vegetables, 2007(03):22.

 微信扫一扫打赏
微信扫一扫打赏

