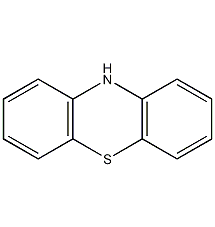
Structural formula
| Business number | 025B |
|---|---|
| Molecular formula | C12H9NS |
| Molecular weight | 199.27 |
| label |
Sulfur-containing nitrogen (hetero)anthracene, 10H-phenothiazine, Thiazaanthracene, diphenylamine sulfide, Thiazaanthracene, Dibenzo-1,4-thiazine, Thiodiphenolamine, Thiodiphenylamine, Longxiang rice, 10H-Phenothiazin, Polymerization inhibitor, pesticides, Heterocyclic compounds |
Numbering system
CAS number:92-84-2
MDL number:MFCD00005015
EINECS number:202-196-5
RTECS number:SN5075000
BRN number:143237
PubChem number:24859973
Physical property data
1. Appearance: yellow to green powder or flake crystal
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 185.5~185.9
5. Boiling point (ºC, normal pressure): 310~312℃ (sublimation at 315℃)
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Spontaneous combustion Point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Not determined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa) : Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
p>
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ether, benzene, acetic acid, chloroform and petroleum, slightly soluble in ethanol, insoluble in water.
Toxicological data
Irritating to eyes, skin, mucous membranes and upper respiratory tract.
Ecological data
None
Molecular structure�Data
1. Molar refractive index: 60.69
2. Molar volume (cm3/mol): 161.5
3. Isotonic specific volume (90.2K ): 435.0
4. Surface tension (dyne/cm): 52.5
5. Polarizability (10-24cm3): 24.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 187
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is easy to oxidize and become darker when stored in the air for a long time, and has sublimation properties. It has a slight odor and is irritating to the skin. Combustible in case of open flame or high heat.
2.Toxic, especially incompletely refined products mixed with diphenylamine, which can be toxic if ingested or inhaled. This product can be absorbed by the skin, causing skin allergies, dermatitis, discoloration of hair and nails, inflammation of the conjunctiva and cornea. It can also stimulate the gastrointestinal tract, damage the kidneys and liver, and cause hemolytic anemia, abdominal pain, and tachycardia. Operators should wear protective equipment. Those who accidentally take it should have their stomach lavaged immediately and get medical treatment.
Storage method
This product should be sealed and stored in a cool, dry place.
Packed in 20kg lined plastic bags, outer woven bags or plastic barrels. Store in a cool, dry and ventilated warehouse. Keep away from moisture and water, sun protection, and away from fire and heat sources. When transporting, load and unload gently to prevent damage to the packaging.
Synthesis method
1. Add diphenylamine, iodine tablets and sulfur into the reaction kettle in sequence, heat with induction for about 4 hours, and raise the temperature to 200 Around ℃. Stir for 2 hours to carry out the vulcanization reaction, then directly heat to 220-250°C with superheated steam. At the same time, blow the reaction product phenothiazine to the receiver, then discharge it to the suction filter, and drain the water under vacuum. The filter cake is washed with a mixture of alcohol and hexamethylenetetramine (mass ratio 1.5:1), dried with hot air, and then crushed to obtain the finished product. The hydrogen sulfide produced during the reaction can be absorbed by sodium hydroxide. The purity of the obtained product is 94% to 97%. The product can be further refined by ethanol recrystallization and activated carbon decolorization.
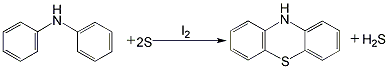
2.Raise the temperature of diphenylamine, sulfonate and sulfur and stir to carry out vulcanization reaction. The moisture of the reaction product phenothiazine was drained under vacuum. The filter cake is washed with a mixture of alcohol and hexamethylenetetramine, dried with hot air, and then crushed to obtain the finished product. The purity of the obtained product is 94% to 97%. It can be further refined using ethanol recrystallization and activated carbon decolorization.
Purpose
1. Phenothiazine is an intermediate for fine chemicals such as drugs and dyes. It itself is an additive for synthetic materials (for the production of vinylon polymerization inhibitors), fruit tree pesticides and veterinary anthelmintics. It has significant effects on gastric worms, nodules, stomatozoal nematodes, Charyx nematodes in cattle, sheep and horses, and thin neck nematodes in sheep. It is used as a polymerization inhibitor for vinyl acetate and vinylon production, and as an additive for synthetic materials such as rubber antioxidants. It is also used in the synthesis of medicines and dyes, as well as anthelmintics for livestock and insecticides for fruit trees. 2.This product is for Used as polymerization inhibitor for vinyl acetate and vinylon production, and as additive for rubber antioxidant and other synthetic materials. It is also used in the synthesis of medicines and dyes, as well as anthelmintics for livestock and insecticides for fruit trees.
extended-reading:https://www.bdmaee.net/dibutyltin-oxide-ultra-pure-818-08-6-cas818-08-6-dibutyloxotin/extended-reading:https://www.bdmaee.net/nt-cat-ea-104-catalyst-cas10027-41-9-newtopchem/extended-reading:https://www.newtopchem.com/archives/974extended-reading:https://www.cyclohexylamine.net/butyltin-trichloridembtl-monobutyltinchloride/extended-reading:https://www.bdmaee.net/nt-cat-e-129-elastomer-catalyst-elastomer-catalyst-nt-cat-e-129/extended-reading:https://www.newtopchem.com/archives/43957extended-reading:https://www.bdmaee.net/dabco-k-15-catalyst-cas3164-85-0-evonik-germany/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/115-11.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2020/07/NEWTOP4.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/38-7.jpg

 微信扫一扫打赏
微信扫一扫打赏

