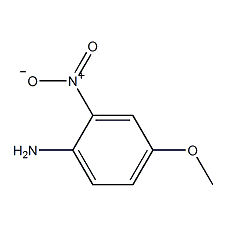
Structural formula
| Business number | 02BN |
|---|---|
| Molecular formula | C7H8N2O3 |
| Molecular weight | 168.15 |
| label |
2-nitro-4-methoxyaniline, 2-Nitro-4-methoxyaniline, 4-Methoxy-2-nitroaniline, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:96-96-8
MDL number:MFCD00007152
EINECS number:202-547-2
RTECS number:BY4415000
BRN number:880318
PubChem number:24896683
Physical property data
1. Properties: Orange-red powder, which can evaporate with water vapor.
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 129
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in cold water, soluble in hot water, ethanol, Ether and dioxane, slightly soluble in benzene.
Toxicological data
Acute toxicity: rat oral LD50: 14100mg/kg
Ecological data
It is extremely harmful to water and toxic to fish. Do not let the product enter the water body.
Molecular structure data
1. Molar refractive index: 43.71
2. Molar volume (cm3/mol): 127.5
3. Isotonic specific volume (90.2K ): 345.2
4. Surface tension (dyne/cm): 53.6
5. Polarizability (10-24cm3): 17.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 81.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 169
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
This product is toxic. For its toxicity and protective methods, please refer to o-nitroaniline (C269).
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packed in iron drums lined with plastic bags. 50kg per barrel. Should be stored in a dry, ventilated place. Avoid sunlight, moisture and heat. Store and transport according to regulations on toxic chemicals.
Synthesis method
1. Method 1: Obtained from nitration and hydrolysis of p-methoxyacetanilide. Add p-methoxyacetanilide and sodium bisulfite to chlorobenzene, slowly add 61% nitric acid at 28°C, first quickly and then slowly within 4 hours. The reaction temperature is controlled at 25-30°C. The addition process Add sodium bisulfite every hour. After the addition is completed, react at 25-30°C for 1.5 hours. Add water, let it sit and then separate the upper acid layer. Add this nitration product to water, add sodium sulfite and adjust the pH value to 7-7.5, and remove chlorobenzene by distillation. Cool to 40°C and add 30% sodium hydroxide solution. Then raise the temperature to 76-77°C within 1 hour and keep it at 77°C for 2 hours. Cool to 30°C, press filter, wash the filter cake with 30°C water until neutral, and dry it. The yield is 80%. Raw material consumption quota: p-methoxyacetanilide (99%) 1629kg/t, chlorobenzene 300kg/t, nitric acid (96%) 590kg/t, liquid alkali (30%) 1300kg/t. Method 2: Use p-aminoanisole as raw material, acidify it with glacial acetic acid, nitrate it with nitric acid and chlorobenzene, then hydrolyze it in the presence of sodium hydroxide, and then filter and dry it. Product specification requirements: content (diazo value) ≥97%, content of impurities insoluble in hydrochloric acid ≤1%, melting point of dry product 121°C. Raw material consumption quota: para-aminoanisole (100%) 1000kg/t, chlorobenzene 450kg/t, glacial acetic acid (98%) 600kg/t, sodium hydroxide (100%) 330kg/t, nitric acid (98%) 660kg /t, sodium metabisulfite 16kg/t.
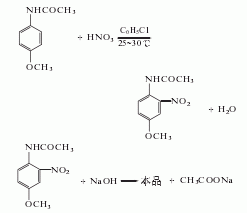
2. Use acetaminophen Methyl ether is used as raw material and is obtained through nitrification, hydrolysis and refining. .
In the nitrification pot, add 750kg of chlorobenzene, 150kg of salt, and 34.4kg of nitric acid (actually 41L, mass 56kg, 63%), then add 300kg of acetaminophen and 103.2kg of nitric acid within 3 hours. (Actually added 132L, mass 168kg, 63%). The reaction temperature was controlled at 20-30°C. After the addition is complete, continue stirring at 30°C for 1 hour. Then add about 31kg sodium carbonate to neutralize to slightly alkaline. Pump the nitrate into the distillation kettle, steam out the chlorobenzene with direct steam, and then reuse it. After distilling off the chlorobenzene, the mixture is left to be hydrolyzed.
Cool the mixture after steaming out the chlorobenzene to 70℃, add 100kg sodium hydroxide (to make a 50% solution), then add water until the volume reaches 1950L, and the temperature drops to 40-50℃. Slowly heat the mixture to 75°C and maintain it at 75-77°C for 2 hours. The feed liquid must remain alkaline. When the melting point of the product reaches 123-124°C, the hydrolysis reaction can be stopped. Cool to 30°C, filter, and wash with water until no alkali is contained. After vacuum drying at 95°C, the crude product was obtained by crushing. The crude product can be recrystallized with hot water to obtain fine product with a melting point of 125-126°C.
Purpose
Used as an intermediate for photosensitive materials. It can also be used as an intermediate for dyes, medicines, and photosensitive materials. It is also used as a raw material for the antimalarial drug primaquinoline in medicine. It is mainly used as the color base of ice dyeing dyes, that is, maroon base GP, used for dyeing cotton, linen, and viscose fabrics and as a color developer for printing. Also used as an intermediate for photosensitive materials. In the pharmaceutical industry, it is used as a raw material for the production of antimalarial drug primaquinoline.
extended-reading:https://www.newtopchem.com/archives/44386extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT4224-catalyst-CAS-68298-38-4-dibutyl-tin-bis-1-thioglycerol.pdfextended-reading:https://www.bdmaee.net/catalyst-pt303/extended-reading:https://www.bdmaee.net/cas-23850-94-4-2/extended-reading:https://www.newtopchem.com/archives/category/products/page/62extended-reading:https://www.newtopchem.com/archives/1824extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Tetramethylpropanediamine-CAS110-95-2-TMPDA.pdfextended-reading:https://www.newtopchem.com/archives/39987extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/59.jpgextended-reading:https://www.cyclohexylamine.net/dabco-ne500-non-emission-amine-catalyst-ne500/

 微信扫一扫打赏
微信扫一扫打赏

