[Background and Overview][1][2]
The English name of methyl 2-bromo-6-bromomethyl benzoate is methyl2-bromo-6-(bromomethyl)benzoate, CAS number is 777859-74-2, and the molecular formula is C9H 8Br2O2, molecular weight 307.96700; PSA: 26.30000; LogP: 3.13060. If methyl 2-bromo-6-bromomethyl benzoate is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing and wash the skin thoroughly with soap and water. If you feel discomfort, Seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
[Structure]
The structure of 2-bromo-6-bromomethylbenzoic acid methyl ester is as follows:
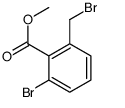
【Synthesis】[2]
Step 1: Preparation of methyl 2-bromo-6-methylbenzoate
Dissolve compound 9 (40g, 186mmol) in thionyl chloride (54.3ml, 744mmol), stir and heat to about 80°C in a 500mL pear flask, and react for about 3 hours. MeOH (200 ml) was added dropwise to the solution in the ice bath through a dropping funnel. The resulting solution was stirred at about 80°C for about 30 minutes. The reaction mixture was diluted with ethyl acetate and saturated NaHCO 3, Wash with water and saturated NaCl, dry the combined organic layers over anhydrous sodium sulfate, filter and concentrate to obtain 2-bromo-6-methylbenzoic acid methyl ester. The reaction equation is as follows:

Step 2: Preparation of methyl 2-bromo-6-bromomethylbenzoate
Combine 2-bromo-6-methylbenzoic acid methyl ester (compound 10, 2.50g, 9.05mmol), N-bromosuccinimide (1.93g, 10.86mmol) and azobisisobutyl A mixture of nitrile (0.669 g, 4.07 mmol) in carbon tetrachloride (20 mL) was stirred at reflux overnight, the mixture was concentrated in vacuo, and the residue was placed on a silica column (PE/EtOAc = 200:1, v/v) After purification, 2-bromo-6-bromomethylbenzoic acid methyl ester was obtained as a white solid (1.62g, yield 50%). The reaction equation is as follows:

[Application][3]
Methyl 2-bromo-6-bromomethylbenzoate can be used as a pharmaceutical intermediate for the synthesis of other compounds with certain activity. Application examples of 2-bromo-6-bromomethylbenzoate methyl ester are as follows:
Combine 2-bromo-6-bromomethylbenzoic acid methyl ester (0.71mmol) and 4-methoxy-3-[2-(4-methyl-piperidin-1-yl)-ethoxy A mixture of ]-aniline hydrochloride (1.5 equiv) in anhydrous DMF (2 ml) was heated to 150 °C in a microwave reactor for 10 min. The mixture is concentrated in vacuo, and the residue is subjected to column chromatography and subsequently converted into the hydrochloride to obtain a new compound with pharmacological activity that can be used to treat CNS and other diseases. The structure is as follows:
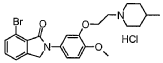
[References]
[1]https://baike.molbase.cn/cidian/2473959?search_keyword=777859-74-2&page=1&per_page=10
[2] GENESTE, Hervé; (DE).OCHSE, Michael; (DE).DRESCHER, Karla; (DE).BEHL, Berthold; (DE).LAPLANCHE, Loic; (DE).DINGES, Jürgen; (US).JAKOB, Clarissa; (US).JANTOS, Katja; (DE) NOVEL INHIBITOR COMPOUNDS OF PHOSPHODIESTERASE TYPE 10AWO2013000994, 28.06.2012
[3] BONANOMI, Giorgio; (IT).HAMPRECHT, Dieter; (IT).MICHELI, Fabrizio; (IT).TERRENI, Silvia; (IT). COMPOUNDS HAVING ACTIVITY AT 5HT2C RECEPTOR AND USES THEREOF. WO2004089897, 05.04.2004

 微信扫一扫打赏
微信扫一扫打赏

