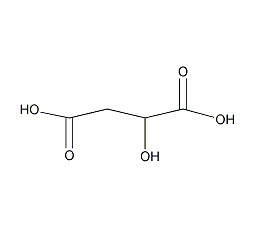
Structural formula
| Business number | 02CK |
|---|---|
| Molecular formula | C4H6O5 |
| Molecular weight | 134.09 |
| label |
S(-)-2-hydroxysuccinic acid, L-butanoldioic acid, L-hydroxysuccinic acid, L-hydroxysuccinic acid, S-(-)-malic acid, L-maleic acid, S(-)-hydroxysuccinic acid, S(-)-2-Hydroxysuccinic acid, S(-)-Malic acid, Natural apple acid, Butanedioic acid, food additives, sour agent, sourness modifier, preserver, pH adjuster, acidic solvent |
Numbering system
CAS number:97-67-6
MDL number:MFCD00064213
EINECS number:202-601-5
RTECS number:ON7175000
BRN number:1723541
PubChem number:24847101
Physical property data
1. Characteristics: colorless crystals with a special fruity sour taste.
2. Density (g/mL, 20℃): 1.60
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 101-103
5. Boiling point (ºC, normal pressure): 306.4
6. Boiling point (ºC, KPa): 140 (decomposition)
7. Refractive index: -6.5
8. Flash point (ºC): 220
9. Specific rotation (º): -2
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg,ºC): Not determined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, soluble in ethanol, ether, Methanol, acetone, insoluble in benzene.
Toxicological data
None
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 25.20
2. Molar volume (cm3/mol): 81.6
3. Isotonic specific volume (90.2K ): 248.9
4. Surface tension (dyne/cm): 86.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. PolarizationRate: 9.99
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -1.3
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
p>
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 94.8
p>
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 129
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with alkalis, oxidants, reducing agents, alkali metals, and amines.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves and smoke.
3. Naturally found in immature apple, hawthorn, and grape juice.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. It should be stored separately from alkali metals, oxidizing agents and reducing agents, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Microorganisms (or enzymes) convert fumaric acid to produce L-malic acid
Microorganisms such as Brevibacterium flavum and Brevibacterium ammoniagenes can all convert fumaric acid. It is malic acid. First, the raw material fumaric acid and calcium carbonate are reacted to generate calcium fumarate, which is then processed through a conversion column (37°C) to generate calcium malate. After extraction, L-malic acid is obtained, and the conversion rate can reach 98%.
2. One-step fermentation of a single strain to produce L-malic acid
Aspergillus oryzae, Aspergillus parasiticus, Aspergillus flavus and other microorganisms can ferment sugar raw materials to produce L-malic acid, using rice sugar As raw material, Aspergillus flavus (ASP Flavus UVT3) was used and fermented in a 500L fermentation tank with ventilation and stirring for 40 hours. The acid production rate was 4.4%~4.95%, and the sugar conversion rate was 68%~75%.
3. Two-step mixed fermentation to produce L-malic acid
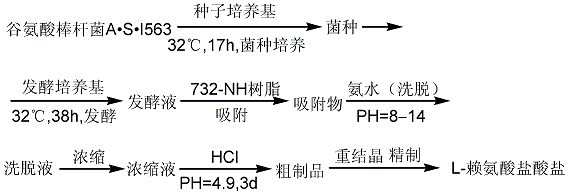
First, the rootless root enzyme R25 is cultured in a medium containing 12% glucose to accumulate fumaric acid , and then inoculate Proteus vulgaris P1 and continue fermentation for 2 days, the production of L-malic acid can reach 5.2~5.48g/100ml, and the sugar conversion rate is 86.7%~91.3%. Using 18% dried potato powder can replace the culture medium containing 12% glucose, and the production of L-malic acid can reach 5.47g/100ml.
The fermentation broth is diluted and filtered. The clear liquid is concentrated under reduced pressure, cooled and crystallized to obtain calcium malate. It is hydrolyzed with sulfuric acid to obtain L-malic acid, which is exchanged with 732 resin. The eluent is then used with 301 anion resin. Exchange the malic acid serum. Finally, the finished product is obtained by concentration under reduced pressure, cooling and crystallization, and recrystallization.
Reaction process:
4. 1. Isolate malic acid from immature apples; industrially, it is mainly produced by catalytic oxidation of benzene to generate maleic anhydride, and then reacting with water under high temperature and pressure.
Purpose
1. Malic acid is an internationally recognized safe food additive used as a sour agent, preservative and pH adjuster. Its sour taste is soft and long-lasting, and its sour taste is 20% stronger than citric acid. It can be used in various types of food and can be used in appropriate amounts according to production needs.
2. Used in the manufacture of esters; used as complexing agents and flavoring agents. Used as food additives and pharmaceutical raw materials.
3. It is widely used in fruity flavors and is a good product for preparing refreshing drinks and ice cream.
extended-reading:https://www.cyclohexylamine.net/pc-37/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/30.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/115-1.jpgextended-reading:https://www.bdmaee.net/bisacetyloxydibutyl-stannane/extended-reading:https://www.newtopchem.com/archives/43968extended-reading:https://www.morpholine.org/soft-foam-amine-catalyst-b16-hard-foam-amine-catalyst-b16/extended-reading:https://www.newtopchem.com/archives/category/products/page/57extended-reading:https://www.bdmaee.net/dbu-octoate-polycat-sa102-niax-a-577/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/23.jpgextended-reading:https://www.newtopchem.com/archives/808

 微信扫一扫打赏
微信扫一扫打赏

