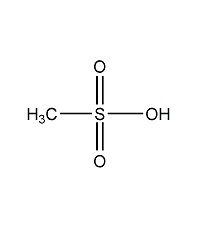
Structural formula
| Business number | 01K0 |
|---|---|
| Molecular formula | CH4O3S |
| Molecular weight | 96.11 |
| label |
Methanesulfonic acid, Methanesulfonic acid, Mesic Acid, Sulfomethane, Aliphatic sulfur compounds |
Numbering system
CAS number:75-75-2
MDL number:MFCD00007518
EINECS number:200-898-6
RTECS number:PB1140000
BRN number:1446024
PubChem number:24873902
Physical property data
1. Properties: colorless or slightly brown oily liquid, solid at low temperatures.
2. Density (g/mL, 25/4℃): 1.4812 (18℃)
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 20℃
5. Boiling point (ºC, normal pressure): 63.7~64.2℃
6. Boiling point (ºC, normal pressure) 5.2kPa): 167℃ (13.33kPa), 122℃ (0.133kPa)
7. Refractive index: 1.4317 (16℃)
8. Flash point (ºC): No Determine
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor Pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
p>
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil-water (octanol/water) partition coefficient The logarithmic value of p>
19. Solubility: Soluble in water, alcohol and ether, insoluble in alkanes, benzene, toluene, etc., does not decompose in boiling water and hot alkali, and has a strong corrosive effect on metal iron, copper and lead.
Toxicological data
1. Acute toxicity
Rat caliber LD50: 200mg/kg
Rat inhalation LC50: >330ppm/6H
Pig skin LD50: > 2mg/kg
Bird caliber LD50: 1mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 17.01
2. Molar volume (cm3/mol): 63.5
3. Isotonic specific volume (90.2K ): 171.8
4. Surface tension (dyne/cm): 53.3
5. Polarizability (10-24cm3): 6.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.9
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 62.8
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 92.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product has a strong irritating effect on the skin and mucous membranes, but is less toxic than methane sulfonic acid.
Storage method
This product should be sealed and stored in a cool, dry place away from light. This product is packed in 250kg plastic drum or steel-plastic drum. Storein a cool, ventilated warehouse. Keep away from fire and heat sources. Store and transport in isolation from oxidants and alkalis.
Synthesis method
It is obtained by oxidation of methyl thiocyanate with nitric acid. Carefully heat the nitric acid and negative water to 80-88°C, add methyl thiocyanate in portions, and the temperature will automatically rise to about 105°C. After the reaction eases, heat to 120°C and react for 5 hours to obtain a crude product. Dilute the crude product with exchange water, add 25% barium hydroxide solution to adjust the pH to 8-9, and filter. The filtrate is shrunk until crystals precipitate, and the crystals are washed with methanol to remove nitrate radicals to obtain barium methane sulfonate. Then add the base to exchange water and boil, add sulfuric acid while it is hot to decompose, filter, and concentrate the filtrate under reduced pressure until no water comes out, and the finished product is obtained. Another preparation method is obtained by chlorination, oxidation and hydrolysis of methylisothiourea sulfate. Add methylisothiourea sulfate to water and introduce chlorine gas at 20-25°C until the color of the solution turns yellow, an oil layer appears at the bottom of the bottle, the temperature drops, and a large amount of residual chlorine is discharged from the exhaust pipe, it is a reaction. end. The reaction solution is extracted with chloroform. After the extract is dried, the chloroform is evaporated under normal pressure at 60-62°C, and then distilled under reduced pressure to collect the 60-65°C (2.67kPa) fraction to obtain methylsulfonyl chloride. Add the base dropwise into 80°C hot water with stirring, and keep warm for hydrolysis for about 2 hours until the oil droplets in the reaction solution completely disappear. The reaction solution is concentrated under reduced pressure until it becomes syrupy, diluted with water, and then concentrated under reduced pressure until no more water can be evaporated to obtain methane sulfonic acid.
Purpose
Methanesulfonic acid is a raw material for medicines and pesticides. It can also be used as a dehydrating agent, coating curing accelerator, fiber treatment agent, solvent, and catalyst for sulfation, esterification and polymerization reactions.
extended-reading:https://www.bdmaee.net/fentacat-100le-catalyst-cas13355-70-2-solvay/extended-reading:https://www.newtopchem.com/archives/44860extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-MP601-delayed-polyurethane-catalyst–delayed-catalyst.pdfextended-reading:https://www.newtopchem.com/archives/44457extended-reading:https://www.bdmaee.net/polyurethane-gel-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/65.jpgextended-reading:https://www.newtopchem.com/archives/43090extended-reading:https://www.newtopchem.com/archives/44752extended-reading:https://www.newtopchem.com/archives/39514extended-reading:https://www.bdmaee.net/dabco-t-45-catalyst-cas121-143-5-evonik-germany/

 微信扫一扫打赏
微信扫一扫打赏

