[Background and Overview][1][2]
7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester CAS number 104146-10-3, chemical formula C24H23ClN2O5S, molecular weight 486.96800, Chinese alias (6R, 7R)-7-phenylacetamido-8-oxo-5-thia- 1-Azabicyclo[4.2.0]oct-2-en-3-chloromethyl-2-carboxylic acid p-methoxybenzyl ester; 3-chloromethylcephalosporin, 3-chloromethyl-7-(2 -Phenylacetamido)-3-cephem-4-carboxylic acid 4-methoxybenzyl ester, boiling point 756.6ºC at 760 mmHg, flash point 411.4ºC, density 1.41 g/cm3, refractive index 1.657, vapor pressure 8.78 E-23mmHg at 25°C. 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester is an intermediate for the synthesis of cefprozil, which is a semi-synthetic broad-spectrum cephalosporin against Gram-positive and Gram-negative bacteria. The activity against Staphylococcus aureus and Streptococcus pneumoniae is better than that of cefaclor, and the stability of penicillinase against Staphylococcus aureus is stronger than that of cefaclor.
7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester has high blood concentration after oral administration, a half-life of about 1.3 hours, a serum protein binding rate of about 45%, and the prototype remains in the urine for 24 hours The recovery rate is about 70% to 80%. CFPZ is well tolerated at doses up to 1000 mg and is primarily eliminated by the kidneys. It is well absorbed after oral administration and exhibits a linear pharmacokinetic relationship. It is suitable for the treatment of pharyngitis, tonsillitis, acute bronchitis, pneumonia, scarlet fever, acute suppurative otitis media, suppurative lymph furunculitis, foreskinitis, furuncle, abscess, staphylococcal syndrome, pustule rash, urinary tract infection, etc. , a promising new oral semisynthetic cephalosporin.
【Synthesis】[1]
A method for preparing 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester, comprising the following steps: adding a reference substance azetidinone sulfenic acid to a reaction kettle , add a chlorinated solvent that is 15 to 25 times the weight of the reference substance, and then add a catalyst that is 1 to 5% of the weight of the reference substance, turn on the circulation pump, circulate the material through the membrane chlorinator, and control the reaction temperature to 20 to 25°C. Air is used as the carrier, and chlorine gas is introduced to carry out the chlorination reaction. When the reaction is completed, the introduction of chlorine gas is stopped, the chlorinated solvent is taken out and recovered, and then the closed-loop solvent is added, and the temperature is controlled at 25~30℃, and the amount of the reference substance is 1.0~1.4mol/mol. Add the ring-closing agent dropwise, add the pH regulator at 20 to 25°C, adjust the pH to 4 to 6, continue stirring, discharge, centrifuge, wash with solvent, and dry to obtain the product 7-phenylacetamido-3-chloromethyl- 4-Cephalosporanic acid p-methoxybenzyl ester. The chlorinated solvent is one or more of 1,4-dioxane, chlorocyclohexane, methylene chloride, and toluene. The catalyst is one or more of 1,2-propylene oxide, 1,3-propylene oxide, 1,2-propylene glycol, and 1,3-propylene glycol. The rate of chlorine gas introduction is 0.3 moles of chlorine per mole of reference material per hour. The amount of carrier air introduced is twice the volume of chlorine. After 3.5 hours, samples are taken and detected by liquid chromatography. When the area of the raw material azetidinone sulfenic acid If it is less than 0.8%, it is considered that the reaction end point has been reached. The chlorinated solvent is extracted and recovered at a vacuum degree of 20mmHg. The closed-loop solvent is one or more of methanol, ethanol, toluene, and methylene chloride, and the dosage is 20 times the weight of the reference substance. The ring-closing agent is a single product or a mixture of sodium methoxide and sodium ethoxide, and the dropping rate is 1 mol/mol of the reference substance per hour. After the dripping is completed, the stirring is maintained for 60 minutes. The weight concentration of the ring-closing agent used is 30%. The pH adjuster is hydrochloric acid, sulfuric acid or acetic acid. The washing solvent is one or more of methanol, ethanol, toluene, and methylene chloride.
[Application][2]
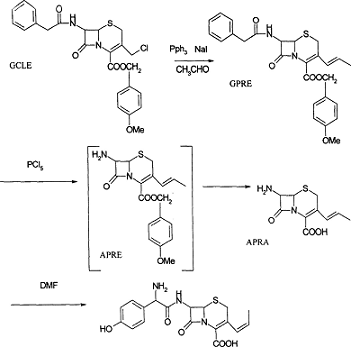
7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester can be used to synthesize the β-lactam antibiotic cefprozil. The preparation method is:
1) Use 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester as the starting material, and mix it with 25-35 grams of sodium iodide in the presence of 10-20 grams of sodium iodide. Phenylphosphine reacts to generate the key intermediate phosphonium salt; temperature: 26~28 ℃; reaction time: 2 hours; solvent: dichloromethane and water;
2) Wittig reaction between the phosphonium salt of step (1) and acetaldehyde produces 7-phenylacetamido-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester; wherein Temperature: -10℃; Reaction time: 16~20 hours; Solvent: methylene chloride;
3) The 7-phenylacetamido-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester of step (2) is mixed with 3 to 8 grams of phosphorus pentachloride, 1 to In the presence of 2ml pyridine and 3~8ml 1,2-propanediol, the 7-position protecting group is removed to generate 7-amino-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester; where temperature: 0 ~10℃; reaction time: 1.5 hours; solvent: methylene chloride;
4) The 7-phenylacetamido-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester of step (3) is mixed with 3 to 8 grams of phosphorus pentachloride, 1 to In the presence of 2ml pyridine and 3~8ml 1,2-propanediol, remove the 7-position protecting group to generate 7-amino-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester; use 15~25ml After m-cresol removes the methoxybenzyl group, 7-amino-3-(prop-1-enyl)-4-cephalosporanic acid is obtained; temperature: -10~-20°C; reaction time: 1.5 hours; solvent: Dichloromethane and water;
5) The 7-phenylacetamido-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester of step (4) is mixed with 3 to 8 grams of phosphorus pentachloride, 1 to In the presence of 2ml pyridine and 3~8ml 1,2-propanediol, the 7-position protecting group is removed to generate 7-amino-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester; in 3~10 Immobilized penicillin acetylaseIt is condensed with p-hydroxyphenylalanine to prepare cefprozil; temperature: 0~5°C; reaction time: 1 hour; solvent: methanol and water.
[Main reference materials]
[1] Zheng Xiaobing; Zhou Xinji; Zhu Jianjun; Zhao Kegui. Method for preparing 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester. CN200810100620.7, application date 2008- 05-12
[2] Chu Xiuhai; Zhu Ye; Ke Hui. Preparation method of oral non-ester antibiotic cefprozil. CN200610024099.4, application date 2006-02-23

 微信扫一扫打赏
微信扫一扫打赏

