[Background and Overview][1][2][3]
2-(4-Fluorophenyl)thiophene CAS number 58861-48-6, chemical formula C10H7FS, molecular weight 178.22600, density 1.201g/cm3 , boiling point 252.316ºC at 760 mmHg, flash point 106.397ºC, refractive index 1.578. 2-(4-Fluorophenyl)thiophene is an intermediate in the synthesis of canagliflozin, and canagliflozin (trade name Invokana) is the first The first approved sodium glucose cotransporter 2 (SGLT2) inhibitor was approved by the US FDA in March 2013 for the treatment of type 2 diabetes. Canagliflozin is one of the most clinically effective new anti-diabetic drugs in recent years. 2-(4-Fluorophenyl)thiophene is an important intermediate in the synthesis of this compound. 2-(4-Fluorophenyl)thiophene is an important intermediate in the synthesis of the anti-diabetic drug Canagliflozin, so the crude product 2-(4-fluorophenyl)thiophene needs to be purified. This compound has special physical properties. It is a white solid at room temperature and a liquid under heating conditions. Therefore, it is difficult to separate and purify. The traditional column chromatography separation method is cumbersome and time-consuming, and is not conducive to industrial production. At present, domestic and foreign literature on the synthesis of this compound only mentions the use of column chromatography to separate the compound. However, this method is cumbersome and difficult to produce industrially.
【Synthesis】[2][3]
Method 1: A synthesis method of 2-(4-fluorophenyl)thiophene, including the following steps:
1) 4-Fluoroacetophenone undergoes Vilsmeier-Haack reaction under the action of solid triphosgene and N,N-dimethylformamide. After adding water for layering, alkalinization, suction filtration, and drying, 3-chloro -3-(4-Fluorophenyl)acrolein;
Further, the molar ratio of 4-fluoroacetophenone, solid triphosgene and N,N-dimethylformamide is: 1:0.4~1.0:1.2~3.0. Further, the reaction system also includes a solvent 5 to 15 times the mass of 4-fluoroacetophenone, wherein the solvent: mass of 4-fluoroacetophenone = mL: g, and the solvent is dichloromethane, chloroform, tetrafluoroacetophenone. Either carbon chloride or 1,2-dichloroethane. Furthermore, the Triphosgene/DMF reagent used in the reaction can also be POCl3/DMF, (COCl)2/DM, SOCl2/DMF The Vilsmeier-Haack reaction is performed instead of other reagents; further, the reaction temperature is 0°C to 25°C.
2) 3-Chloro-3-(4-fluorophenyl)acrolein undergoes a ring-closing reaction with ethyl thioglycolate under the action of alkali. After adding water and filtering, 5-(4-fluorophenyl) is obtained. ) Thiophene-2-carboxylic acid ethyl ester; further, the ester group part of the ethyl thioglycolate in this reaction can be substituted by a C1 to C5 straight chain or branched chain alcohol, and the ethyl thioglycolate or ester group part is The molar amount of substituted ethyl thioglycolate is 1 to 1.5 times that of 3-chloro-3-(4-fluorophenyl)acrolein. Further, the molar amount of base is 1 to 3 times that of 3-chloro-3-(4-fluorophenyl)acrolein. The base can be an inorganic base, such as sodium carbonate, potassium carbonate, sodium ethoxide, sodium methoxide, potassium hydroxide, sodium hydroxide, lithium hydroxide, sodium tert-butoxide, potassium tert-butoxide, sodium tert-amyloxide, potassium tert-amyloxide etc.; it can also be organic bases such as triethylamine, pyridine, etc. Further, the reaction system also includes a solvent 3 to 10 times the mass of 3-chloro-3-(4-fluorophenyl)acrolein, wherein the solvent: 3-chloro-3-(4-fluorophenyl)acrolein Mass=mL:g, the solvent is any one of NMP, DMF, DMAc, pyridine, acetonitrile, acetone, 1,4-dioxane, tetrahydrofuran, methanol, ethanol and isopropyl alcohol. Further, the reaction temperature is 30°C to 80°C.
3) 5-(4-Fluorophenyl)thiophene-2-carboxylic acid ethyl ester undergoes ester hydrolysis under the action of alkali. After adding water, acidification, suction filtration, and drying, 5-(4-fluorophenyl) is obtained. ) Thiophene-2-carboxylic acid; further, the base is an inorganic base that is 1.0 to 2.0 times the number of moles of 5-(4-fluorophenyl)thiophene-2-carboxylic acid ethyl ester, and can be lithium hydroxide, sodium hydroxide, Any one or more of potassium hydroxide, sodium carbonate and potassium carbonate. Further, the reaction system of step 3) also includes a solvent 3 to 10 times the mass of 5-(4-fluorophenyl)thiophene-2-carboxylic acid ethyl ester, wherein the solvent: 5-(4-fluorophenyl) Mass of ethyl thiophene-fluorophenyl)thiophene-2-carboxylate = mL: g. The solvent is any one of methanol, ethanol, isopropyl alcohol, propanol, and 1,4-dioxane.
4) 5-(4-fluorophenyl)thiophene-2-carboxylic acid is heated and decarboxylated under the catalysis of copper powder, followed by filtration, crystallization, suction filtration and recrystallization to obtain 2-(4-fluorophenyl) Thiophene. Further, the amount of copper powder used is 10% of the mass of 5-(4-fluorophenyl)thiophene-2-carboxylic acid. Further, the solvent used in this reaction includes dimethyl sulfoxide, sulfolane, quinoline, etc.; the volume used is 2-10 times the mass of the substrate; further, the temperature used in the reaction is 160°C-250°C.
Method 2: A method for synthesizing 2-(4-fluorophenyl)thiophene, including the following steps:
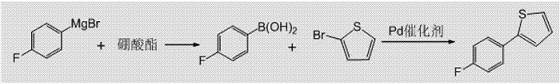
1) Preparation of 4-fluorophenylboronic acid: Pre-incubate the 4-fluorophenyl magnesium bromide solution as material 1 and the borate ester solution as material 2 to the reaction temperature respectively, and then follow the steps of 4-fluorophenyl bromide Mix magnesium chloride and borate ester at a molar ratio of 1:1.0 to 1:2.5, react at -10°C to 20°C, and then perform quenching and post-processing to obtain 4-fluorophenylboronic acid;
2) Preparation of 2-(4-fluorophenyl)thiophene: Add 4-fluorophenylboronic acid, 2-bromothiophene and Pd catalyst into an organic solvent and dissolve to obtain material 3, in which: 4-fluorophenylboronic acid is in The molar concentration in the organic solvent is 0.5mol/L~2mol/L, the molar ratio of 4-fluorophenylboronic acid and 2-bromothiophene is 1:1.0~1:1.5, and the molar ratio of 4-fluorophenylboronic acid and catalyst is 1 :0.005~1:0.1;��Material 3 and the inorganic alkali aqueous solution as material 4 are pre-insulated to the reaction temperature and then mixed according to the molar ratio of 4-fluorophenylboronic acid and alkali is 1:1.0~1:3.0, at 50℃~80℃ The reaction is carried out under the conditions, and then post-treatment is performed to obtain 2-(4-fluorophenyl)thiophene. The synthesis route is as follows:
[Application][3]
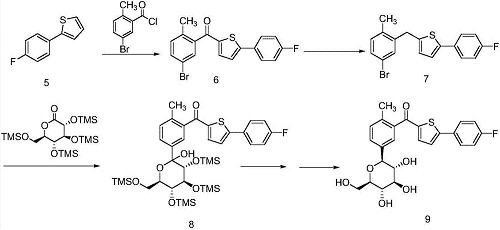
2-(4-Fluorophenyl)thiophene can be used as an intermediate to synthesize canagliflozin. For example, 2-(4-fluorophenyl)thiophene can be used as raw material to perform acylation, reduction, and alkylation reactions to prepare canagliflozin. After listing, the reaction equation is as follows:
[Main reference materials]
[1] Meng Yanqiu; Liu Wenhu; Ding Yi; Yang Zhe. A method for separation and purification of 2-(4-fluorophenyl)thiophene. CN201310592895.8, application date 2013-11-22
[2] Yang Kun; An Yongsheng. A method for synthesizing 2-(4-fluorophenyl)thiophene. CN201610223793.2, application date 2016-04-11
[3] Li Xiaoliang; Zhang Bisheng; Chen Lingling. A synthesis method of 2-(4-fluorophenyl)thiophene. CN201511009947.X, application date 2015-12-29

 微信扫一扫打赏
微信扫一扫打赏

