[background and overview][1][2][3]
p-toluenesulfonyl chloride (4-methylsulfonyl chloride, often abbreviated as toscl or tscl) is a chemical with the molecular formula ch3c6h4 so2cl is an organic compound. this compound is a white solid reagent with a foul smell and is widely used in organic synthesis. this compound is a derivative of toluene and sulfonyl chloride functional groups. p-toluenesulfonyl chloride is a white flake crystal with a melting point of 69-71°c. it is an important organic synthesis drug intermediate and is mainly used in the synthesis of chloramphenicol, chloramphenicol-t, thiamphenicol and other drugs. p-toluenesulfonyl chloride is an important fine chemical product, which is widely used in the dye and pharmaceutical industries. it is used as an intermediate for the manufacture of ice dyes, disperse dyes and organic pigments; in the pharmaceutical industry, it is an intermediate for more than ten kinds of antibacterial and anti-inflammatory drugs such as betamethasone and mesulfonate; it is also used as a plasticizer for the preparation of plastics. intermediates for agents, resins, coatings, pesticides and photosensitive materials. market prospects. the currently commonly used synthesis method of p-toluenesulfonyl chloride is mainly the toluenesulfonylation method. the reason why this method has become the main method for producing toluenesulfonyl chloride today is because this process has the characteristics of few operating steps and short reaction cycle, but at the same time, there are the ortho- and para-isomers are difficult to separate and the product quality is poor. there are currently two main purification methods in common use, one is the freezing separation method, and the other is the vacuum distillation method. the so-called freeze separation method is to place the obtained mixed sulfonyl chloride at a temperature of -20-0°c. after freezing for a period of time, the para product will crystallize and precipitate, and the ortho position will be oily. finally, it can be separated by a centrifuge. the vacuum distillation method separates ortho- and para-isomers based on their boiling point difference at a pressure of 1.388-2.660 kpa. neither method yields high-purity products. another synthetic route is the sodium toluenesulfonate chlorination method. this method is to sulfonate toluene with concentrated sulfuric acid and then perform a chlorination reaction with thionyl chloride. the traditional sulfonation method of concentrated sulfuric acid produces a large amount of waste sulfuric acid, and using thionyl chloride as the chlorinating agent is relatively expensive. therefore, it is of great social and economic significance to improve the yield of toluene chlorosulfonation reaction, reduce environmental pollution, and reduce production costs.
【synthesis】[1][2][3]
method 1: synthesis of p-toluenesulfonyl chloride using toluene, chlorosulfonic acid and phosphorus oxychloride as raw materials and ammonium chloride as catalyst:
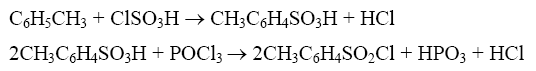
in a 250ml four-necked flask equipped with a stirrer, reflux condenser, and thermometer, add a certain amount of chlorosulfonic acid according to the raw material ratio at room temperature, add the catalyst while stirring, and then add toluene dropwise; after addition, use after the oil bath is automatically heated to 75°c, phosphorus oxychloride is added dropwise, then the temperature is raised to 105~110°c, and the reaction ends under constant temperature stirring for a certain period of time. cool the reaction solution to 70~80°c and then pour it into a mixture of ice and water to proceed with hydrolysis, crystallization and vacuum filtration. the filter cake is dried under vacuum at constant temperature and then weighed to obtain the p-toluenesulfonyl chloride product; the filtrate is extracted with solvent oil, washed twice with water, the water layer is separated, the oil layer is collected, and the sulfonated oil mainly composed of o-toluenesulfonyl chloride is obtained through vacuum distillation. . the optimal raw material molar ratio: toluene: chlorosulfonic acid: phosphorus oxychloride: ammonium chloride is 1:1.3:0.6:0.1, the product yield reaches 98.85%, and the pure yield is 97.86% (p-toluenesulfonyl chloride 85.41% , o-toluenesulfonyl chloride 12.45%); at the same time, the acidic wastewater is reduced by more than 70% compared with the original process.
method 2: p-toluenesulfonyl chloride was synthesized using toluene as raw material. in the sulfonation reaction, the azeotropic water-removing sulfuric acid sulfonation method is used. in the chlorination reaction, carbon tetrachloride is used as the solvent and chlorine gas is used as the chlorinating agent. this process has high sulfuric acid utilization rate and low production cost. the steps are as follows:
1) synthesis of p-toluenesulfonic acid: in a four-stage furnace equipped with a constant pressure funnel, stirrer, condenser and thermometer
in the flask, add an appropriate amount of toluene, raise the temperature to 105-110°c, slowly add 27 ml (0.5 mol) of concentrated sulfuric acid dropwise, and keep it at the boiling point temperature for 5 hours. during this period, the water generated by the reaction will and toluene vapor is continuously steamed out, and then enters the oil-water separator after condensation. the dehydrated toluene is then dripped into the reactor through the dropping funnel to maintain excess toluene during the sulfonation process. after the reaction is completed, excess toluene is evaporated, the temperature is cooled, the reactant is poured into an appropriate amount of water, p-toluenesulfonic acid crystallizes out, filtered, washed, and dried to obtain 80.6 g of product, with a yield of 93.72%.
2) synthesis of p-toluenesulfonyl chloride: equipped with stirrer, thermometer, gas distributor, condenser and
in the four-necked flask of the gas recovery device, add 50 ml of carbon tetrachloride, 17.2 g of p-toluenesulfonic acid, and 1.5 g of sulfur powder. stir and raise the temperature to 60°c. start to introduce chlorine gas, and control the ventilation rate at 3-3.5 g/h, close the ventilation valve after 10 hours, stir for 1 hour, and then vent chlorine gas for 5 hours. after the reaction, the solvent was recovered and the reactants were poured into ice water. filter and air-dry to obtain 14.7 g of p-chlorobenzenesulfonyl chloride, with a yield of 85.47%.
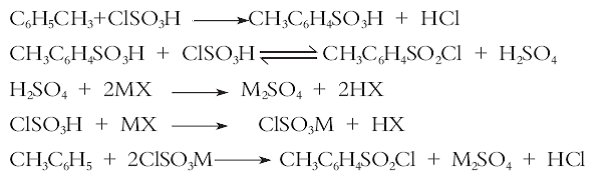
method 3: using toluene as the starting material, introducing carbon tetrachloride diluent, selecting a composite catalyst, performing an acid chlorination reaction with chlorosulfonic acid to synthesize p-toluenesulfonyl chloride, and using a new process of direct crystallization, purification and separation using solvents. compared with the traditional process, this process has the advantages of high yield, good product content and good selectivity, reduces the dosage of chlorosulfonic acid, increases the concentration of waste acid, provides convenience for the comprehensive utilization and treatment of waste acid, and is suitable for industrial production. the reaction equation is as follows:
[application][3]
p-toluenesulfonyl chloride, as a fine chemical product, is widely used in the dye, pharmaceutical, and pesticide industries. in the dye industry, it is mainly used to manufacture intermediates for dispersed, ice dyeing and acid dyes; in the pharmaceutical industry, it is mainly used to produce sulfa drugs, mesulfonate, etc.; in the pesticide industry, it is mainly used in the production of mesotrione, sulfotrione, fine metalaxyl, etc. with the continuous development of the dye, pharmaceutical and pesticide industries, the international demand for this product is growing day by day, especially in europe and the united states, where the market prospects are broad.
[main reference materials]
[1] hao yanxia, su yanxi. research on the synthesis of p-toluenesulfonyl chloride[j]. hebei chemical industry, 2006, 29(6): 17-18.
[2] chen zhongxiu, jiang linfeng, ding tongfu, et al. research on the synthesis of p-toluenesulfonyl chloride[d]. , 2004.
[3] xu yun, wang hongliang, jin xingbei, et al. research on the synthesis of new process of p-toluenesulfonyl chloride[j]. zhejiang chemical industry, 2003, 34(10): 10-11.

 微信扫一扫打赏
微信扫一扫打赏

