[Background and Overview][1]
N-Methyl-4-nitrophenylethylamine hydrochloride, its CAS number is 166943-39-1, N-methyl-4-nitrophenylethylamine hydrochloride chemical formula C9H13ClN2O2, molecular weight 209.28600, Melting point 222-227ºC, boiling point 335.8ºC at 760 mmHg, vapor pressure 8.36E-05mmHg at 25°C. If inhaled, move the patient to fresh air; in case of skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if eye contact occurs, separate the eyelids and rinse with fluid Rinse with water or saline and seek medical attention immediately. If ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
[Application]
N-Methyl-4-nitrophenylethylamine hydrochloride is a pharmaceutical intermediate that can be used to synthesize other compounds with certain biological activity such as dofetilide. Dofetilide is an oral antiarrhythmic drug developed by Pfizer of the United States for atrial fibrillation. It has a high effect of reversing sinus rhythm. It was first launched in the United States in May 2000 and is a Class III antiarrhythmic drug. The antiarrhythmic dofetilide is disclosed in European patent EP-A-0245997, and its patent family members include US4959366 (divisional application US5079248), CN1014529, etc. This published patent describes various synthesis methods of dofetilide. However, there are problems such as raw materials being difficult to obtain and industrial production costs being high. Dofetilide is a methanesulfonamide derivative that is structurally similar to the antiarrhythmic drug sotalol. Its pharmacological mechanism of action is to exert the effect of Class III antiarrhythmic drugs by inhibiting the rapid potassium current (Ikr) and increasing the action potential duration (QT interval). It was approved by the U.S. Food and Drug Administration (FDA) in 1999 and was first launched in the United States in May 2000. A large number of clinical studies have shown that dofetilide can be used to treat and prevent atrial rhythm disorders and paroxysmal supraventricular tachycardia, and is more effective than other antiarrhythmias in converting newly developed atrial fibrillation and atrial flutter. medicine. It can also be used to prevent the occurrence of ventricular tachycardia and relieve the condition of patients with heart failure. It has the advantages of significant curative effect and relatively small adverse reactions. It is a powerful and selective anti-arrhythmic drug. N-Methyl-4-nitrophenylethylamine hydrochloride is an important intermediate in the synthesis of dofetilide. Examples of its applications are as follows:
1. Use N-methyl-4-nitrophenylethylamine hydrochloride free base as raw material, introduce a methanesulfonamide group, then dock and reduce, and finally introduce another methanesulfonamide group, Finally, the final product dofetilide is obtained, and the reaction equation is as follows:
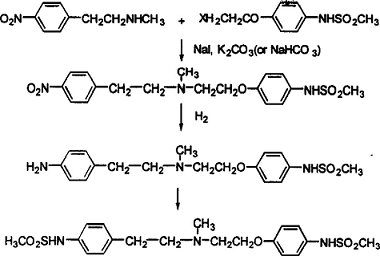
2. Using N-methyl-4-nitrophenylethylamine hydrochloride free base as raw material, dinitro compounds are first synthesized, and then reduced and then subjected to mesylation reaction to finally obtain the final product Dofei Li Te, the reaction equation is as follows:
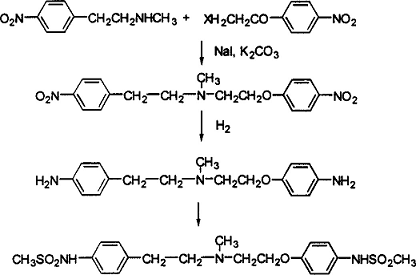
[Main reference materials]
[1] Gao Chuanfu; Gao Xuesong New method for preparing dofetilide. CN02117190.4, application date 2002-04-25

 微信扫一扫打赏
微信扫一扫打赏

