[Background and Overview][1][2][3]
Meta-aminophenol (m-Aminophnol, MAP) is a widely used fine chemical raw material. With the development of fax technology and agricultural technology, the demand for m-aminophenol, as a colorless pressure/heat-sensitive material, pesticide intermediate, and API, is increasing year by year. In 2012, China’s MAP consumption was as high as 4,000t; the largest foreign consumer of m-aminophenol is Japan, whose consumption in 2013 was close to 2,500t. In recent years, due to the control of environmental pollution and water and soil pollution, various environmental protection policies have been introduced one after another. Many domestic MAP production plants have withdrawn from the project. Therefore, it is urgent to find out the MAP green production route. In medicine, it can be used to make p-aminosalicylic acid, a good drug for treating tuberculosis. In the dye industry, m-aminophenol can be used to make N, N-trimethyl-3-aminophenol, which is used as an intermediate for mordants and basic dyes and as a phase developer; it can be used to make 3-anilinophenol, which is used as an coupling agent Nitrogen dye intermediate, used to make N,N-diethyl-3-aminophenol, used as various dye intermediates and anti-corrosion agent for jet fuel; used to make fur dyes such as fur brown, fur yellow, etc. In addition, m-aminophenol can also be used as a catalyst for organic chemical reactions, such as the polymerization of propylene oxide and the dimerization of acrylonitrile, etc. It can also be used as a metal anti-corrosion agent and alloy herbicide and insecticide in alkaline media, and is used to produce anti-oxidants. Oxygen, stabilizer, developer, etc.
Abroad, m-aminophenol is mainly used to produce dyes and pesticides. For example, in Japan, 78% of its domestic consumption is used for dyes, of which 70% of dye consumption is used to produce functional colorless dyes and traditional dyes. Consumption is only 30%. In particular, the production of colorless pressure/heat-sensitive dyes occupies an absolutely dominant position. With the development and popularization of fax technology, the market demand for colorless pressure/heat-sensitive dyes is developing rapidly. From the mid-1980s to the early 1990s, the average annual growth rate of this type of dye in Japan was as high as 26.6%. In recent years, the growth rate has slowed down, but it is still a major area of growth in the world’s demand for meta-aminophenol. In addition, the market demand for traditional dye varieties produced from m-aminophenol has basically remained stable.
【Synthesis】[1]
1. Nitrobenzene reduction method
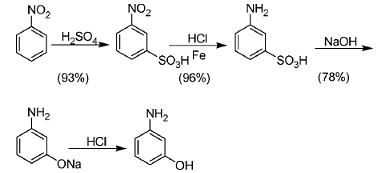
This craft is a traditional craft, and only India and China continue to use it. The main problem of the process is the processing of large quantities of waste acid, waste alkali and iron sludge. In addition, the high temperature conditions of the alkali smelting process increase production costs and can easily lead to personal accidents.
2. Metaphenylenediamine method
1) Direct hydrolysis of m-phenylenediamine: In 1980, a process route for the synthesis of MAP by hydrolyzing m-phenylenediamine in dilute hydrochloric acid was developed for the first time. The following is the reaction equation:
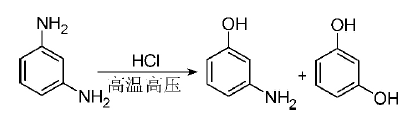
The large concentration of hydrochloric acid is very corrosive to reactors and other equipment, and the long reaction time restricts production capacity, so it has not been industrialized. Some domestic scholars have optimized the process and used sulfuric acid as a catalyst to reduce the corrosiveness of the equipment.
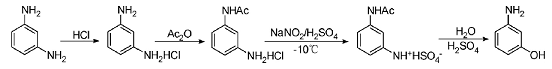
2) Synthesis method through the intermediate meta-aminoacetanilide hydrochloride: acidify the monoamino group of m-phenylenediamine to form a salt, selectively protect the amino group by acetylation, and use NaNO2Carry out diazotization and dilute sulfuric acid hydrolysis to finally obtain MAP.
3. Aniline hydroxylation method
After the reaction, MAP, o-aminophenol and p-aminophenol can be obtained. The yield of MAP is not high, about 64%.
4. Electrochemical reduction method
Constant current electrolysis of m-nitrophenol can be reduced to obtain MAP.
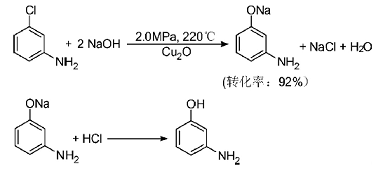
5. Pressurized alkaline hydrolysis of m-chloroaniline
Using cuprous oxide as a catalyst and high-pressure alkaline hydrolysis of m-chloroaniline to prepare MAP, the reaction formula is as follows:
6. Resorcinol aminolysis method
The ammonia hydrolysis route was first proposed by Japanese scholars and studied in depth. Its by-product is only water, with less three waste emissions and a short process route. It is favored by MAP manufacturers.
1) Gas-phase ammonolysis method: The gas-phase ammonolysis method was developed by Japan’s Mitsui Petrochemical Company in 1987.
At high temperatures, ammonia and resorcinol gases are passed into the catalytic bed, and the by-products in the reaction cause the catalyst to lose activity. Despite experiments using various catalysts, resorcin conversion and MAP selectivity were both low.
2) Liquid phase ammonolysis method
Liquid phase ammonolysis mechanism: Different from the chemical properties of other phenols, resorcinol has similar properties to α-naphthol, β-naphthol and their derivatives, and can perform Bucherer reaction with ammonia in an autoclave.
A: No catalyst: non-catalytic reaction. The reaction formula is as follows:
B: Inorganic salt catalyzed ammonolysis
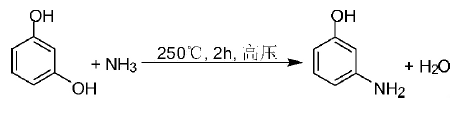
However, it is difficult to separate the inorganic salts using inorganic salt catalysis.
C: Aluminosilicate catalyzed ammonolysis: Certain aluminosilicates have a very good promotion effect on the catalytic ammonolysis reaction, such as LZM-8 and LZM -62, and their silicon to aluminum ratio is generally 2 to 100. The dosage of catalyst is 10% to 100% of the weight of the reactants, and the MAP yield is still above 80% after being recycled 5 times. This process significantly reduces the production cost of the catalyst in catalytic ammonolysis.
[Application][2][3]
Meta-aminophenol (MAP), also known as m-hydroxyaniline, has a wide range of uses.
1. Pharmaceutical: Mainly used in the production of anti-tuberculosis drug sodium p-aminosalicylate. From 1993 to the present, my country’s sodium aminosalicylate production capacity has basically remained at 500 to 600 tons, but the output is only about 100 tons, and more than 70 tons of m-aminophenol are consumed. Since tuberculosis bacteria are prone to develop resistance when sodium aminosalicylate is used, domestic tuberculosis is decreasing day by day, and it is expected that the output will not increase significantly in the next few years.
2. In terms of dyes: domestically, it is mainly used to produce the dye intermediate m-hydroxy N, N-diethylaniline, and then synthesize a variety of dyes and coumarin fluorescent whitening agents. At present, m-hydroxy N, N-diethylaniline in my country The output of m-hydroxyaniline is about 2,000 tons/year, but some domestic companies still use sodium m-nitrobenzene sulfonate as raw material to produce m-hydroxy N, N-diethylaniline due to factors such as the high price of m-aminophenol and high production costs. This limits the application development of m-aminophenol to a certain extent. If the cost of domestic m-aminophenol can be reduced and the price is further reduced, the consumer market of m-aminophenol in dyes can be effectively opened up. Only m-hydroxy N, N-diethylaniline consumes approximately 1,400 yuan of m-aminophenol every year. Ton. With the eastward shift of the world’s dye industry production and trade center, another major consumption channel for my country’s m-aminophenol is export. At present, more than 60% of my country’s total m-aminophenol production is exported. As an important organic intermediate, especially as an important functional dye intermediate, m-aminophenol has good development potential and market prospects. However, the current technology used to produce m-aminophenol in my country is backward, resulting in a large amount of Three wastes, polluting the environment. Today, with increasingly stringent environmental protection requirements, the production of aminophenol by nitrobenzene sulfonation and alkali dissolution has no future. Therefore, the pace of technological transformation should be accelerated so that the new process of synthesizing m-aminophenol by resorcinol aminolysis can be industrialized in my country as soon as possible.
3. Pesticide industry: Mainly used for the production of betanin and betaine, the synthetic raw material of m-hydroxyphenyl ethyl carbamate. Other uses are smaller, such as the six-step synthesis of naphquinate, a quinoline anti-coccidial drug, through acylation, rearrangement, benzylation, reductive hydrolysis, condensation, and cyclization; the Friedlander reaction involves the intermediate N-acetyl Meta-aminophenol is used to synthesize the fungicides fenacetamine and fluoxanine.
5. Others: In recent years, as composite materials and polymeric resins have become more and more widely used, MAP is used as a monomer to undergo a condensation reaction to prepare graphene/nickel oxide-poly(aniline-m-aminophenol) for supercapacitors. Composites and triglycidyl-m-aminophenol epoxy resins for biosensors. The synthesized composite material has stronger conductivity, smaller electrode conductivity, better capacitance performance, and better cycle stability; in addition, MAP has dual properties of diamine and amine, and is highly carcinogenic. If it enters drinking water, it will pose a serious threat to human life and health. MAP in water is removed through chlorination and oxidation.
[Main reference materials]
[1] Dong Anzhou, et al. Preparation method of m-aminophenol and progress of Bucherer reaction. Dyes and Dyeing, 2016, 1: 43-47.
[2] Cui Xiaoming. Meta-aminophenol has a wide range of uses. China Petroleum and Chemical Industry, 2000, 5: 48-48.
[3] Yang Qingqing. Research on the process of preparing resorcinol from meta-aminophenol. Dalian University of Technology, 2017

 微信扫一扫打赏
微信扫一扫打赏

