[Background and Overview][1][2][3]
L-p-Methyldibenzoyltartaric acid monohydrate CAS number 71607-31-3, chemical formula C20H20O9, molecular weight 404.37, Chinese alias L-DTTA monowater, white or off-white crystalline powder, melting point 163-165ºC, boiling point 686ºC at 760 mmHg, flash point 368.7ºC. If L-p-methyldibenzoyl tartaric acid monohydrate is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and if you feel discomfort, Seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
【Synthesis】[1]
The synthesis of L-p-methyldibenzoyltartaric acid monohydrate is ultimately the synthesis of L-p-methyldibenzoyltartaric acid. The specific method is as follows: a kind of L-p-methyldibenzoyltartaric acid The synthesis method is simple, safe and easy to operate, and the process yield reaches more than 95%. At the same time, the raw material cost is low, and some raw materials can be recycled and recycled. The finished product has high purity and excellent chiral separation performance. The synthesis method is: first use L-tartaric acid, p-methylbenzoyl chloride and thionyl chloride as raw materials, use toluene as the solvent, react to prepare L-p-methyldibenzoyltartaric anhydride, and then add L-p-methyldibenzoyltartaric anhydride. methyl dibenzoyl tartaric anhydride is hydrolyzed by adding water to obtain L-p-methyl dibenzoyl tartaric acid. The reaction process is as follows:
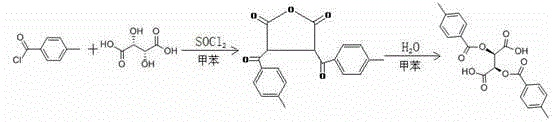
The specific synthesis steps are:
1) Put 1 part of L-tartaric acid and an appropriate amount of organic solvent by weight into the reaction kettle. Under stirring conditions, add 0.1-0.001 parts of catalyst into the reaction kettle, and then add 1-10 mL/min Add 1 to 3 parts of p-toluoyl chloride into the reaction kettle at a dropping rate of 100%. After the dropwise addition is completed, continue the reaction for 6 hours. Then, transfer the reaction mixture to a centrifuge for centrifugal separation to obtain solid L -p-methyldibenzoyltartaric anhydride, for later use;
The catalyst is copper sulfate or ferrous sulfate;
2) Put the L-p-methyldibenzoyltartaric anhydride prepared in step 1) into the reaction kettle, add equal weights of toluene and water to it, and heat to reflux at 100°C for 4-6 hours , after that, the temperature is lowered to normal temperature, and the obtained mixture is transferred to a centrifuge for centrifugal separation to obtain solid L-p-methyldibenzoyltartaric acid.
[Application][1][2]
L-p-Methyldibenzoyltartaric acid is a white crystalline powder that easily absorbs water in the air and becomes L-p-methyldibenzoyltartaric acid monohydrate, which is soluble in organic solvents such as ethanol and methanol. . Widely used for chiral resolution of amine compounds. And it has good industrial application value because of its low price, stable properties and easy recycling. L-p-Methyldibenzoyltartaric acid monohydrate is widely used for chiral separation of racemic amine compounds. For example, it is used in the synthesis of tapentadol hydrochloride and its analogues. Tapentadol hydrochloride is the hydrochloride of tapentadol. It is a new type of central analgesic with dual action mechanisms developed by Johnson & Johnson of the United States. Pain medicine. It is used as medicine in the form of a single isomer of (1R, 2R). The synthesis method is to use 1-(3-substituted phenyl)-1-ketone compounds as starting materials, and undergo reduction, halogenation, nucleophilic substitution, After hydrolysis, decarboxylation, amidation, reduction, resolution and hydrochloric acid salt formation, the compound tapentadol hydrochloride is obtained, wherein the chiral resolving reagent is selected from chiral organic acid compounds (such as D or L-malic acid, L-lactic acid), D or L-type amino acid compounds (such as L-lysine, L-proline, D-cysteine, D-phenylalanine, D-tryptophan, D-valine acid, etc., D or L-tartaric acid), replacing D or L-type tartaric acid (such as D-(+)-p-methyldibenzoyltartaric acid, L-p-methyldibenzoyltartaric acid or L-p-methyldibenzoyltartaric acid) Benzoyltartaric acid monohydrate, D‑(+)‑dibenzoyltartaric acid, L‑(‑)‑dibenzoyltartaric acid, D‑p‑methoxydibenzoyltartaric acid or L‑p‑methoxy Dibenzoyl tartaric acid, etc.), D or L type camphorsulfonic acid and D or L type mandelic acid, etc.
[Main reference materials]
[1] Liu Hang; Zhang Daowei; Li Zhijun; Chu Jingbo; Cui Qinghai; Cui Fei; Li Yamin; Wang Jianxun; Li Rong; Fan Weibin; Zheng Jishuan; Yuan Guolong; Wen Jiaogang; Zhang Jianmin. A kind of L-p-methyldiphenyl Synthesis method of acyl tartaric acid. CN201410836127.7, application date 2014-12-30
[2] Xu Ziao; Zhao Yonghai; Li Xiaoxiang; Li Degang. A new synthesis method of tapentadol hydrochloride and its analogs. CN201110413051.3, application date 2011-12-13

 微信扫一扫打赏
微信扫一扫打赏

