[background and overview][1]
benzenedione (benzoyl) is widely used in organic synthesis and pharmaceutical industry as a raw material and intermediate. it can be used to synthesize pesticides, photosensitizers for uv-curable resins, and printing inks for food, etc. it has broad application prospects in the pesticide, pharmaceutical and food industries.
[preparation method][1]
benzoin is used as raw material to prepare benzoethylene glycol. traditional oxidation methods include: chromate oxidation, nitric acid oxidation, permanganate oxidation, ferric chloride oxidation, copper sulfate oxidation, etc. these oxidation methods all use metered oxidants, which are expensive, have a lot of waste residue, and cause serious pollution. it is of great significance to develop a green and efficient catalytic oxidation method to catalytically oxidize benzoin to prepare benzophenone.
molecular oxygen is cheap and abundant. it is very attractive to develop an efficient catalytic system using molecular oxygen as the oxygen source for selective oxidation of benzoin to prepare benzoate. journal of molecular catalysis a: chemical(1996,111,33‑36) reported a method using co(acac)2, fe(acac)3a method for catalyzing the oxidation of benzoin with oxygen to prepare benzoate. this method directly uses oxygen as the oxidant. the conversion rate of benzoin and the selectivity of benzoate are relatively high, but during the reaction process, it is necessary to add a certain amount of raw materials. benzoin contains three times the amount of aldehydes or acetals, which increases the cost and increases the difficulty of product separation and purification. journal of molecular catalysis a: chemical (2001,165, 283-290) discloses an oxygen species as an oxidizing agent, h3+npmo12‑nvno40 (n=1 ‑4)as a catalyst to catalyze the oxidation of benzoin to prepare benzoate, the reaction conditions are relatively mild. the by-products such as benzaldehyde, benzoic acid and benzoate produced due to the breakage of carbon-carbon bonds make benzoate diketones are not very selective. catalysis communications (2010,11,684‑688) reported that air was used as an oxidant and hydrotalcite doped with different metals was used as a catalyst to catalyze the oxidation of benzoin. the reaction conditions were mild and the selectivity of benzoethylene glycol was very good. , but the catalyst preparation steps are cumbersome and the cycle is long.
a method of directly using molecular oxygen to oxidize benzoin to prepare benzoate, specifically using a bismuth compound as a catalyst, oxygen or air as an oxidant to oxidize benzoin to prepare benzoate, and a bismuth compound as a catalyst it has low toxicity, the oxidant oxygen comes directly from the air, low cost, and the reaction conditions are mild, so it has good industrial application prospects.
add 0.1 mol% (relative to the molar amount of raw material benzoin) bismuth oxide and 0.53 g benzoin into a 10 ml three-necked flask, add 2 ml absolute ethanol, introduce oxygen by bubbling, and raise the temperature to 80°c with stirring. and keep it for 6h. then cool to room temperature, take samples and compare the gas chromatography retention time of gc-ms and standard materials to identify the oxidation products. the main product detected by gc-ms is benzoethylene glycol, as well as a small amount of benzaldehyde and benzoic acid. the gas chromatography retention time is the time comparison results were consistent with gc‑ms and were quantitatively analyzed using gas chromatography. the reaction schematic diagram is as follows: formula 1, and the corresponding analysis chromatogram is shown in figure 1. the conversion rate of benzoin is 96%, the selectivity of benzoate is 94%, and the by-products are mainly a small amount of benzaldehyde and benzoic acid.
[application][2] [3]
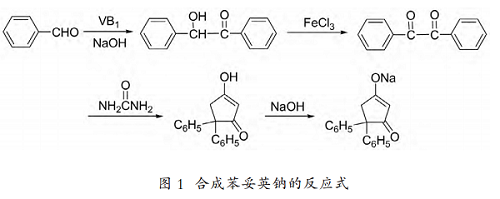
phenytoin (sodium phenytoin) chemical name is 5,5-diphenyl-2,4-imidazolidinedione sodium salt, also known as dalentin sodium, which is the drug of choice for anti-epileptic grand mal and localized seizures. , often used for anti-epilepsy, anti-arrhythmia, anti-trigeminal neuralgia, and anti-mild hypertension. the main synthesis method of phenytoin sodium: benzaldehyde is condensed with benzoin under the catalysis of vitamin b1 to obtain benzoin, benzoin is oxidized to produce benzoethylene glycol, benzoethylene glycol reacts with urea under alkaline conditions to obtain phenytoin, and finally phenytoin reacts with hydrogen sodium oxide is converted into salt to obtain phenytoin sodium. benzaldehyde is synthesized from benzoin under the catalysis of vitamin b1, and then oxidized with ferric chloride to produce benzoethylene glycol, which is then reacted with urea to obtain phenytoin, and finally salted with sodium hydroxide to form phenytoin sodium. the reaction formula for synthesizing phenytoin sodium is as follows:
benzophenone can be used to prepare inks. ink is printingindispensable chemical raw materials in the field of technology. ultraviolet light curing (uv) ink refers to ink that uses ultraviolet light of different wavelengths and energies to film and dry the ink under ultraviolet irradiation. different uv spectra can be used to generate different energies to polymerize monomers in different ink binders into polymers, so the color film of uv ink has good mechanical and chemical properties. therefore, light-curing offset printing ink is a direction worthy of research in the industry.
a light-curing offset printing ink consists of the following components by weight: 40-50 parts of epoxy acrylic resin, 20-25 parts of polyester acrylic resin, and 18-20 parts of trimethylolpropane triacrylate. , 55-60 parts of tripropylene glycol diacrylate, 10-12 parts of benzophenone, 8-9 parts of benzophenone, 5-6 parts of methyldiethanolamine, and 48-55 parts of magenta. according to the following weight ratio: epoxy acrylic resin 40kg, polyester acrylic resin 25kg, trimethylolpropane triacrylate 20kg, tripropylene glycol diacrylate 55kg, benzophenone 12kg, diphenylethylene glycol 9 kg of ketone, 5 kg of methyldiethanolamine, and 55 kg of magenta are used to make light-curing offset printing ink. when printing, it dries quickly, prints clearly, is water-resistant, solvent-resistant, and has better wear resistance than traditional light-curing offset printing ink. .
[main reference materials]
[1]cn201110413541.3
[2]li gongchun, wu changzeng, guo junwei, sun ting, niu liangfeng, ju zhiyu. synthesis of phenytoin sodium [j]. zhejiang chemical industry, 2015, 46(08): 23-25.
[3] cn201510313306.7

 微信扫一扫打赏
微信扫一扫打赏

