[Overview]
Resorcinol, also known as 1,3-benzenediol, m-dihydroxybenzene, commonly known as resorcinol, is an important fine chemical raw material. Resorcinol is prone to reactions such as hydrogenation, halogenation, amination, acylation, coupling, alkylation, nitration and sulfonation, making it widely used in agriculture, dyes, coatings, medicine, plastics, rubber, electronic chemicals and other fields. Has a wide range of applications. Resorcinol is a basic organic chemical intermediate, widely used in agriculture, medicine, adhesives, etc. In medicine, resorcinol has a bactericidal effect and can also be used to prepare intestinal disinfectants and skin disease drugs; in agriculture, resorcinol is used to prepare agricultural films, pesticides, herbicides, etc.; in dyes In medicine, resorcin is used to synthesize fluorescent dyes and acidic eosin; in adhesives, resorcin is used to produce resorcin-formaldehyde resin and special thermosetting wood adhesives, which are used for gluing high-end furniture. It is also an industry with rapid growth in usage; in addition, resorcinol can also be used to produce explosives, liquid crystal polymers, ultraviolet absorbers, etc.
[Physical and chemical properties]
The appearance is white needle-like crystals, with a weak aromatic odor, slightly sweet and bitter taste, a density of 1.272g mL, a boiling point of 281°C, a melting point of 110.7°C, and a flash point of 127.1°C (closed cup). It can sublimate at high temperatures, is flammable, is soluble in water, ethanol, ether, glycerin and amyl alcohol, and is insoluble in chloroform and carbon tetrachloride. Resorcinol is a dibasic weak acid that can react with bases to form monophenolates and bisphenolates. Resorcinol is slightly acidic but more acidic than carbonic acid. It will gradually turn light red when exposed to sunlight and air for a long time. Resorcinol is more easily oxidized than phenol, but the rate of oxidation by oxygen or periodic acid is much lower than that of catechol and hydroquinone. Resorcinol is prone to reactions such as hydrogenation, halogenation, amination, acylation, coupling, alkylation, nitration and sulfonation.
[Preparation method]
1. Sulfonation method The typical production process of resorcinol sulfonation method is: Benzene is sulfonated in fuming sulfuric acid to generate benzenesulfonic acid, and further sulfonated to generate isophthalic acid, and the isophthalic acid produced is The sulfonic acid mixture is melted with NaOH alkali to generate sodium resorcinol, which is neutralized with acid to obtain resorcinol.
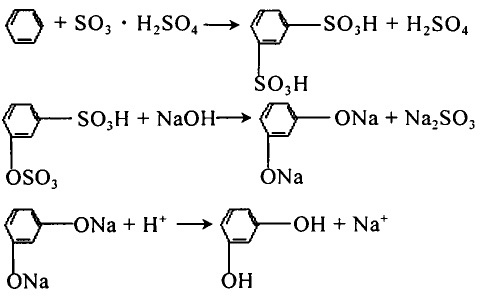
Figure 1 is the reaction equation of resorcinol sulfonation method
2. Cumene method The synthesis of resorcinol by cumene method is carried out in three steps: 1) Alkylation of benzene, that is
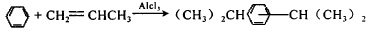
Figure 2 shows the reaction equation for the alkylation of benzene 2) Oxidation of cumene
![]()
Figure 3 shows the reaction equation for the oxidation of cumene 3) Acidolysis of peroxide
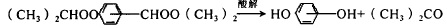
Figure 4 is the reaction equation for the acidolysis of peroxide
This method generally involves purifying the alkylation product and then oxidizing it using pure m-dicumylbenzene as the raw material. It can also be carried out without purification using a mixture of para and meta positions as the raw material. At this time, the cracked product is a mixture of benzoquinone and resorcinol, which must be further separated. The separation of resorcinol and benzoquinone generally uses the crystallization method first. The crystallization uses organic solvents such as propionitrile, methanol, toluene, ethers, ethyl acetate, etc., and the filter cake is benzoquinone. Resorcinol is in the filtrate. After multiple recrystallizations, the obtained filter cake is very pure benzoquinone. The purity of resorcinol in the filtrate is not enough, and it can be further purified by steam distillation. 3. Aromatization method Aromatization method uses cyclohexanedione-1,3 to prepare resorcinol through dehydrogenation. There are two preparation methods. One is to use cyclohexanedione-1,3 and acetic anhydride under the catalysis of sulfuric acid to prepare resorcinol diester and then hydrolyze it. The reaction formula is as follows:
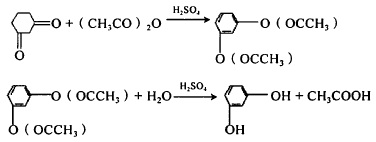
Figure 5 shows the reaction equation of aromatization method
(1) Another method is to directly dehydrogenate cyclohexanedione-1,3 at high temperatures above 170°C in the presence of a dehydrogenation catalyst such as Pd.
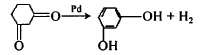
Figure 6 is the reaction equation of aromatization method
(2) 4. Group substitution method (1) Nitrogen substitution: Use o-chlorophenol as raw material, hydroxylate it with KOH in o-dichlorobenzene, and react at 170°C. Under these conditions, the reaction generates catechol and partially rearranges to generate resorcinol. The reaction product contains 57.5% resorcinol and 36% catechol.
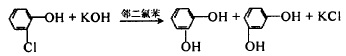
Figure 7 is the reaction equation of the nitrogen substitution method
(2) Substitution of nitrogen groups. Using m-phenylenediamine as raw material, m-phenylenediamine is prepared through radical substitution. The reaction formula is:
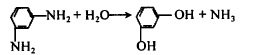
Figure 8 is the reaction equation of the nitrogen group substitution method
【Purpose】
Resorcinol is aIt is an important organic chemical product and synthetic intermediate and has a wide range of uses. In tires, hoses and tapes, resorcinol-formaldehyde resin (RF resin) is used to impregnate rayon and polyamide fiber cords to increase the adhesion between the cords and rubber. RF resin can be cured at room temperature and neutral conditions, is not corroded by water, and will not cause decomposition of the adherends. Therefore, in addition to being widely used in the rubber industry, it can also be used as an adhesive for synthetic fibers, wood, metal, ceramics, plastics, etc. Treating steel cords with resorcinol-triaryl cyanurate-formaldehyde resin can improve the bonding properties of steel wires to rubber. Adding resorcinol, hexamethylenetetramine and silica to rubber compounds can improve the reinforcing effect of short fibers in the rubber matrix.
Due to the fast polycondensation reaction and curing speed of resorcinol and formaldehyde, it can be used to modify phenolic resin and urea-formaldehyde resin, which can accelerate the curing of the resin and make the product have better water resistance. Bis(diphenylphosphonate)resorcin ester synthesized from resorcinol is a non-halogen flame retardant with high thermal stability and is suitable for modified polyether, polycarbonate, ABS resin and various thermoplastic resins and polyurethane and other plastics. Resorcinol has a certain bactericidal effect and is often used to make ointments and apply externally to treat skin diseases caused by fungal infections; aerogels made from resorcinol and formaldehyde can be used as electrodes in chemical batteries and used as salty wastewater. Or when seawater passes through the electrode, a large number of ions can be removed from the water, so that salty wastewater can be treated or seawater can be desalinated. Ether compounds prepared from resorcinol can be used as stabilizers and plasticizers for cellulose ethers or esters, cross-linking agents for epoxy resins and unsaturated polyesters, and are also important chemical reagents.
2,4-Dihydroxybenzylketone, produced by the condensation of resorcinol and acetaldehyde, can be used as an intermediate for the preparation of “ethoxyflavonoids”, a drug for the treatment of coronary heart disease. 2,4-dihydroxyphenylethyl ketone, produced by the condensation of resorcinol and propionic acid, can be used in the pharmaceutical industry to prepare the neurostimulant drug “Huisulin”. 2,4,6-trinitro-resorcin, which is obtained by trinitration or nitrosation and then oxidation of resorcin, can be used as explosives and propellants containing nitrocellulose. 2,4-dihydroxybenzoic acid produced by the reaction of resorcinol and carbon dioxide is an intermediate for the preparation of dyes, photosensitive materials and cosmetics. As an intermediate in the pharmaceutical industry, it is often used in the synthesis of bactericidal drugs and anti-rheumatic drugs. In the dye industry, instead of salicylic acid as the coupling component, azo dyes with darker color and higher intensity can be synthesized than those using salicylic acid as the coupling component. In addition, it can also be used to prepare various halogenated, nitrated or sulfonated products of resorcinol.
Meta-aminophenol, synthesized from resorcinol, can be used medicinally to produce para-aminosalicylic acid (PAS), a good drug for treating tuberculosis. In the dye industry, it can be used to make N,N-dimethyl-3-aminophenol, which is used as a mordant and basic dye intermediate and photographic developer; it can be used to make 3-anilinophenol, an azo dye intermediate, and to make fur. Dyes such as fur brown, fur yellow, etc. In addition, it can also be used as a catalyst for the polymerization of propylene oxide and the dimerization of acrylonitrile, as a metal anti-corrosion agent in alkaline media and in the production of herbicides, insecticides, antioxidants, stabilizers and developers, etc. . 7-hydroxyvanillin, synthesized from resorcinol and maleic anhydride, has antifungal, bactericidal, antibacterial and choleretic activities and can be used in pharmaceutical ointments; it can also be used to prepare antihypertensive drugs. In addition, it can also be used as a fluorescent whitening agent for plastics, textiles, soaps, and detergents. 4-Methyl-7-hydroxycoumarin, obtained by the reaction of resorcinol and ethyl acetoacetate, can be used to mask the yellow color in soaps, detergents, paraffin, plastics, fibers and papers, and can also be used as a UV Shielding agent. In addition, resorcin can also be used for fur dyeing and hair dye, as a chemical analysis reagent, as a raw material for the synthesis of plastic polybenzobisoxazole, azo dyes, fluorescent dyes and acid eosin. Synthetic agricultural films, synthetic insecticides (pyrifosin), herbicides (ethoxyfluorfen, etc.) and intestinal disinfectants, etc.
[Main reference materials]
[1] Cui Xiaoming. Production, application and market prospects of resorcinol [J]. Sichuan Chemical Industry and Corrosion Control, 2002(05):41-44.
[2] San Yu. Exploration on the development of low-pollution synthesis process of resorcinol [D]. Chongqing University, 2003.
[3]Dai Zilin, Lu Yi, Wei Qing, Xiao Benyi. Synthesis method of resorcinol [J]. Journal of Wuyi University (Natural Science Edition), 2000(02):24-28.

 微信扫一扫打赏
微信扫一扫打赏

