Background and overview[1][2]
Methyl phenylpropionate is also known as methyl phenylpropionate, methyl dihydrocinnamate, and methyl hydrogenated cinnamate, CAS number 103-25-3, chemical formula C10H 12O2. Molecular weight 164.20100, density 1.043g/mL at 25°C (lit.), vapor pressure 0.0423mmHg at 25°C, boiling point 91-92°C4 mm Hg (lit.), flash point 212°F, refractive index 20/D 1.502 (lit.), white crystal. Methyl phenylpropionate can be used as an intermediate in organic synthesis.
If methyl phenylpropionate is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eyes are clear In case of contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
Preparation[2]
Dissolve phenylpropionic acid in excess anhydrous methanol. Anhydrous methanol serves as both a reaction reagent and a reaction solvent. Add excess thionyl chloride dropwise, heat to reflux and stir for 3-4 hours to remove the solution. The crude product is distilled under reduced pressure to obtain methyl phenylpropionate.
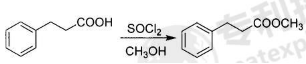
Purpose[2]
Methyl phenylpropionate is used as a pharmaceutical intermediate and also in organic synthesis. Examples of its application are as follows:
Used in the synthesis of methyl p-sulfonyl chloride phenylpropionate. Aromatic sulfonyl chloride is an important pharmaceutical intermediate (synthetic sulfonamide drugs) with broad application prospects. Methyl phenylpropionate is used to synthesize methyl phenylpropionate of p-sulfonyl chloride. The process is simple and practical, easy to operate, the raw materials are easily available, and the cost is low. The technical solution adopted by the present invention to achieve the above object is: the synthesis method of methyl phenylpropionate of sulfonyl chloride, first esterify the phenylpropionate, then sulfonate it with chlorosulfonic acid, then hydrolyze, dry the precipitated solid, and repeat The product is crystallized and refined. The specific process is:
The first step of esterification: Dissolve phenylpropionic acid in excess anhydrous methanol. Anhydrous methanol serves as both a reaction reagent and a reaction solvent. Add excess thionyl chloride dropwise, heat to reflux and stir the reaction 3- After 4 hours, the solution was removed, and the crude product was distilled under reduced pressure to obtain methyl phenylpropionate; the molar ratio of benzoic acid to thionyl chloride was 1:1.0-2.0.
The second step of sulfonation: add excess chlorosulfonic acid to the reaction flask, add the methyl phenylpropionate prepared in the first step to the chlorosulfonic acid at low temperature, stir and raise the temperature to 50-70°C, and react for 2-8 hours , react at room temperature for 6-14 hours; the molar ratio of methyl benzoate and chlorosulfonic acid is 1:3-8.
The third step of hydrolysis: pour the sulfonation reaction solution into ice water for hydrolysis, control the temperature below 5℃, dry the precipitated solid, and then recrystallize and refine it to obtain the product.
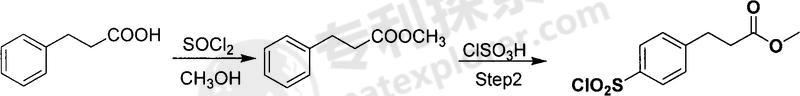
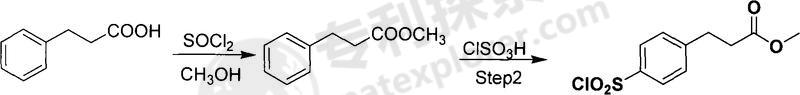
Compared with similar synthesis methods, this method has significant characteristics: the raw materials used are cheap and easy to obtain, the reaction process route is short, the process conditions are easy to control, and the final product obtained has excellent performance, high purity, and high yield. , suitable for industrial production and meeting the quality requirements of pharmaceutical production.
Main reference materials
[1] Compound Dictionary
[2] Wang Rongliang; Jiang Renwu. Synthesis method of methyl p-sulfonyl chloride phenylpropionate . CN200910011199.7 , application date 20090417

 微信扫一扫打赏
微信扫一扫打赏

