Background and overview【1】
Elavirenz, chemical name (4S)-6-chloro-4-(cyclopropacetylene)-4-(trifluoromethyl)-benzo-1,4-dihydroxazole- 2-one, developed by the American company Merck, is a specific drug against HIV. It belongs to the category of non-nuclear reverse transcriptase inhibitor drugs. It is mainly used for human immune virus type A (HIV type 1). It was launched in 1999 In February, it was approved for sale by the Food and Drug Administration (FDA) of the United States under the trade name Sustiva. Cyclopropylacetylene (1) is the key intermediate for the synthesis of efavirenz. The existing processes at home and abroad mainly include cyclopropyl methyl ketone, cyclopropyl methyl aldehyde, 5-chloro-1-pentyne and other A synthesis method containing alkynyl compounds as raw materials.
Efavirenz is an anti-HIV infection drug. It is never used alone, but is combined with other drugs to form a compound. The following factors should be considered when deciding to start drug treatment: CD4 reading, HIV source HIV viral load, patient’s diagnosis and treatment history, drug resistance and personal wishes. In 2006, initial results from the ACTG 5142 trial were released, which compared efavirenz to lopinavir. Efavirenz was used as a first-line treatment for various protease inhibitors. ACTG 5095 trial showed that efavirenz was more effective in maintaining CD4 readings. Potential for HIV pathogenic load.
Efavirenz belongs to the non-nucleoside reverse transcriptase inhibitor (NNRTI — non-nucleoside reverse transcriptase inhibitor) category of antiretrovirals. Both nucleoside-based RTIs and non-nucleoside-based RTIs are intended to inhibit The same target, reverse transcriptase enzyme. This enzyme transcribes RNA into DNA and is extremely important for virus reproduction. The difference between NNRTI and nucleoside-based RTI is that the former is bundled in the NNRTI sheath, while the latter is bundled in the active surface of the enzyme body. Efavirenz is not effective against HIV type 2. The reason is that the structure of the reverse transcriptase in the HIV-2 sheath is different. Therefore, it has inherent resistance to NNRTI drugs.
Since all NNRTIs are bundled in the same NNRTI sheath, viral strains that are resistant to efavirenz are also resistant to other NNRTIs (such as nevirapine and delavirdine). When using The most common viral mutation after drug treatment is the K103N type, and this mutation is usually also observed after the use of other NNRTI drugs. Side effects are often accompanied by the following psychopathological symptoms: insomnia, confusion, confusion, memory loss, depression, etc.; rash, nausea, dizziness and headache may also occur; efavirenz may cause birth defects, so it should not be used The safety of use on infants in women of potential pregnancy has not been confirmed; use of efavirenz may also result in falsely positive urine tests for marijuana substances.
Application
Used as an antiviral drug and a non-nucleoside reverse transcriptase inhibitor. Used in combination with other viral reverse transcriptase inhibitors for the treatment of HIV-1 infected patients.
Preparation【1】
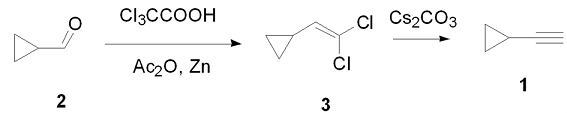
Considering the availability of raw materials and cost, the preparation method used before oral use is to use cyclopropyl methyl ketone as raw material and react with phosphorus pentachloride at low temperature to generate 1,1-dichloro-1-cyclopropyl Ethane is then used to obtain cyclopropylacetylene under the action of a base. This reaction has many by-products and the yield is only 34%-42%. The literature method uses cyclopropylformaldehyde as raw material, reacts with trichloroacetic acid, vinegar, and zinc powder to obtain 1-(2, 2-dichlorovinyl)cyclopropane, 1-(2, 2-dichlorovinyl)cyclopropane, Propane is eliminated under strong alkali conditions to obtain 1. The yield of this reaction has been greatly improved, but the flammable and explosive reagent methyl cannium needs to be used in the reaction, which is not conducive to industrial production. In order to simplify the synthesis process of 1, reduce the difficulty of synthesis, and improve the yield, this article optimized the synthesis process of 1 based on the literature, using carbonic acid instead of methyl cannium reagent, and optimizing the amount of alkali and reaction conditions. That is, cyclopropylmethylaldehyde (2) is used as raw material, reacts with trichloroacetic acid, vinegar and zinc powder to obtain 1-(2,2-dichlorovinyl)cyclopropane (3), 3 is reacted with carbonic acid 1 was synthesized through the reaction, with a total yield of 80% (see Figure 1 for the synthesis route), and its structure was confirmed by H NMR and C NMR.
1. Experimental part
1. 1 Main instruments and reagents
Experimental reagents: trichloroacetic acid (Aladdin’s reagent), acetic acid preparation (Keyi reagent), other reagents used are analytical grade or chemical grade, Broker 400MHz nuclear magnetic resonance instrument (neon chloroform is the solvent, TMS is the internal standard).
1.2 Experimental steps
1.2.1 Synthesis of 3
Stir cyclopropylmethylaldehyde (30 g, 0.43 mol), trichloroacetic acid (105g, 0.64 mol) and DMF (300 mL) at room temperature, then add sodium trichloroacetate (119 g , 0.64 mol), after the addition is completed, continue the reaction at room temperature for 5 hours, then cool to 0-5°C, add acetic acid (80.8 mL, 0.86 mol), continue stirring for 1 hour, add 400 mL of acetic acid to dilute, and then add under nitrogen protection Zinc powder (56.0 g, 0.86mol), the reaction temperature was raised to 60°C for 1 hour, cooled to room temperature, 300 mL of water was added, extracted three times with 500 mL of hexane, the organic phases were combined, and washed once with 500 mL of water. Then wash once with 500 mL of saturated sodium chloride solution, let it stand, separate the layers, dry the organic phase with anhydrous magnesium sulfate, filter, concentrate, and evaporate under reduced pressure.��Product 3 (52 g, 88%) was obtained.
1.2.2 Synthesis of 1
Add 3 (20 g, 146 mmol), toluene (20 mL), and carbonic acid (71.5 g, 219 mmol) into the reaction flask, heat to 110°C and react for 10 hours. After the reaction is completed, cool to room temperature and add cold water. Wash and dilute with 50 mL, extract 3 times with 50 mL of n-hexane, combine the organic phases, wash once with 50 mL of water, and then wash once with 50 ml of saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and distill the filtrate to obtain the product Cyclopropylacetylene 8.5 g, 90%, H NMR 8 (CDC13): 0.65-0.75 (m, 4H), 1. 15-1.22 (m, 1H), 1. 71 (s , 1H); C NMR 8 (CDC13): 0.90, 7.95, 63.3, 87.5.
Main references
[1]Wu Gaoxin, Zheng Yunfeng, Luo Hairong. Optimization of the synthesis process of efavirenz intermediates[J]. Shandong Chemical Industry, 2018, 47(14):18-19.

 微信扫一扫打赏
微信扫一扫打赏

