Chlorphenesin
In medicine, chlorphenesin-related preparations are antigen-related immunosuppressants that inhibit IgE-mediated histamine release. Chlorphenesin is also an antifungal drug. It is suitable for antifungal, bacterial, vaginal mold, and trichomonas diseases. There are many pharmaceutical dosage forms.
In terms of cosmetics, chlorphenesin is used as a cosmetic bactericide in the “International Dictionary and Manual of Cosmetic Raw Materials”. Chlorphenesin can effectively resist Gram-positive and -negative bacteria, especially Aspergillus niger IMl149007 and Philophila Penicillium pine IMI87160 (fungus) has strong bactericidal activity and has a good inhibitory effect on Candida albicans NCPF3179 and Saccharomyces cerevisiae NCPF3275 (yeast). In the past ten years, chlorphenesin has been used abroad to manufacture medicated toothpaste, nail polish, soap, soap, shampoo products, and skin care products.
Preparation of chlorphenesin
Regarding the preparation of chlorphenesin, there are few reports at home and abroad. Most of them are prepared by chemical methods. One method uses p-chlorophenol and epichlorohydrin as raw materials. The reaction principle is as follows
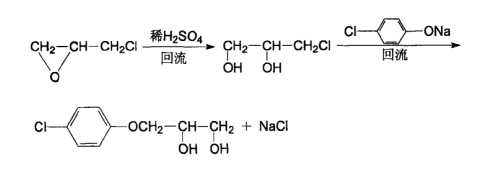
This method uses ethanol and water as recrystallization solvents for post-processing, and high-purity products can be obtained.
Another method is to use p-chlorophenol and glycidyl alcohol as raw materials and pyridine or quaternary ammonium salt as the catalyst. The reaction formula is as follows
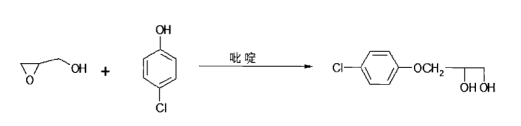
The crude product obtained by this method can be recrystallized with a mixed solvent of diethyl ether and petroleum ether, or can be recrystallized with chloroform to obtain high-quality products.
Currently, because the raw material of glycidyl alcohol is not easy to obtain, the first method is mostly used to prepare chlorphenesin. This method uses sulfuric acid as the oxidant and is carried out in two steps. It is relatively complicated, but the yield is high. The crude product yield reaches 90%, the recrystallization yield reaches 80-95%, and the reaction conditions are relatively easy to control.
Reaction between p-chlorophenol and chlorinated glycerin
It can be seen from the above information that the reaction of p-chlorophenol and chlorinated glycerin is a preparation method of chlorphenesin, and it is also a relatively common preparation method.

 微信扫一扫打赏
微信扫一扫打赏

