Background[1][2]
The molecular formula of dichlorobenzene is C6H4Cl2. There are three isomers: ortho, meta and para. Usually mainly o- and p-dichlorobenzene. O-dichlorobenzene is a colorless liquid with a pungent odor and can be used as a solvent for grease, resin, rubber, etc., as well as a pesticide. Acute poisoning: Rats were exposed to LD502138mg/kg orally and died within 7 hours of exposure to a concentration of 977ppm, mainly due to damage to the liver and kidneys and disorders of hematopoietic organs.
P-Dichlorobenzene is a solid that has a pungent smell and can sublimate. It is commonly used as insect repellent, deodorant, insecticide, fumigant and organic synthesis intermediate. Acute toxicity: Rat oral LD502512mg/kg. Occupational exposure averaged 105ppm, and workers suffered from eye and nose irritation symptoms and lung, liver and kidney disorders. Para and ortho bodies are equally carcinogenic. The maximum allowable concentration in the air of the working environment: p-dichlorobenzene is 75ppm (450mg/m3) in the United States and 50ppm (300mg/m3) in Japan; o-dichlorobenzene The US regulation for benzene is 50ppm (300mg/m3).
Dichlorobenzene enters the environment in various forms such as wastewater, waste gas, garbage and waste residues without proper treatment, causing pollution to different environmental media. They have been listed as priority pollutants for testing by the U.S. Environmental Protection Agency. A large part of the pollution of this type of compounds in farmland soil comes from irrigation water. The soil and groundwater in some sewage irrigation areas in my country have been polluted by this type of compounds to varying degrees. When crops are polluted, it not only affects their yield and quality, but can also be enriched through the food chain and endanger human health.
Apply[3][4]
Dichlorobenzene is widely used in chemical, textile, electronics, medicine, manufacturing and other industries as an organic solvent, insecticide, disinfectant, dye, pesticide, and organic synthesis intermediate. For example, p-dichlorobenzene is an important raw material for organic chemical synthesis. It is widely used as a pesticide intermediate, fumigation insecticide, fabric mothproofing agent, antifungal agent, and air deodorant. Most of it is used in the manufacture of sanitary pellets. , such as 32% of paradichlorobenzene used as deodorant in the United States.
Japan’s p-dichlorobenzene used as deodorant and moth-proofing agent accounts for 70% of its total production capacity; an important use of p-dichlorobenzene is as an intermediate for high-performance engineering plastics polyphenylene sulfide, polyphenylene sulfide Sulfide has excellent properties such as high temperature resistance, radiation resistance, ablation resistance, high toughness and high stability, and is used in the fields of electronics, electrical, machinery manufacturing and aerospace; p-dichlorobenzene can also be used to synthesize 2,5-dichloronitrate Benzene is an intermediate for the synthetic dyes big red base GG and red base 3GL, active bright yellow and red RC, etc. It is also a new antibacterial agent for medicine trichlorosine (2,4,4-trichloro-2-hydroxydiphenyl ether) The main raw material; a small amount of p-dichlorobenzene is also used in special pressure lubricants and corrosion inhibitors. This product can also be used as a solvent, mutagen, etc.
O-dichlorobenzene (1,2-dichlorobenzene) is an excellent solvent that can be used as a solvent for wax, gum, resin, tar, rubber, oil and asphalt. It is used in the dyes Shilin black and Shilin. The solvent 1,2-dichlorobenzene is used in the production of forest yellow brown, high-end pigments, the drug chlorhexidine, and polyurethane raw material TDI; this product can be used to make insecticides for termites, locusts, and borers, and for trichlor insecticide It can also be used in the production of esters, threocysterol, and xinyanling, and can also be used to synthesize catechol, fluorochloroaniline, 3,4-dichloroaniline and o-phenylenediamine.
As an anti-rust agent and degreasing agent, o-dichlorobenzene can remove carbon and lead from engine parts, remove coatings on metal surfaces without corroding the metal, and can remove sulfur from lighting gases. It can be used as an ingredient in metal polishing agents; o-dichlorobenzene dyes are also used in the industry to make vat blue CLB and vat blue CLG; polymer wet spinning solvents to reduce fiber thermal shrinkage; o-dichlorobenzene can also be used as epoxy Resin diluent, coolant, heat exchange medium. Production of long-acting sulfonamides for medicines, etc.
Biodegradation[3]
Due to its relatively stable chemical properties and biological toxicity, it is difficult to be naturally degraded by microorganisms after entering the soil environment as an environmental foreign matter. Through enrichment, it can easily cause persistent pollution to the environment. Moreover, dichlorobenzene has a strong irritating effect. Long-term exposure will cause irritation to human skin, conjunctiva and respiratory organs, and can cause acute or chronic neurological disorders. Therefore, the three isomers of dichlorobenzene are all listed as priority monitoring substances by the US EPA, and 1,2-dichlorobenzene and 1,4-dichlorobenzene are also priority monitoring substances in my country.
After dichlorobenzene enters the soil, it is restricted by its own physical and chemical properties, soil multi-media system characteristics and external environmental conditions. It mainly experiences (1) volatilization into the atmosphere, (2) adsorption and desorption by soil particles, ( 3) Leach into groundwater, and (4) be biodegraded, among other processes. These processes often occur simultaneously, and each process is interrelated and affects each other.
For dichlorobenzene remaining in the soil, biological degradation is a more thorough treatment method, and the possibility of secondary pollution is minimal. Although dichlorobenzene is a refractory organic pollutant, it can still be biodegraded under aerobic conditions by cultivating special microorganisms and utilizing co-metabolism. Dichlorobenzene is a synthetic environmental foreign compound. Generally, microorganisms in nature lack the degradative enzymes required to degrade dichlorobenzene. When there is a lack of appropriate microbial populations in the soil, dichlorobenzene is difficult to be biodegraded.
But in recent years, many researchers haveThrough artificial domestication and natural domestication, a variety of microbial strains that can degrade dichlorobenzene have been isolated, which can partially or completely degrade dichlorobenzene. Compared with aromatic hydrocarbons, the biodegradability of dichlorobenzene is greatly reduced, mainly because the high electronegativity of the chlorine atom of its substituent strongly attracts the electrons on the benzene ring, making the benzene ring an electron-phobic ring that is difficult to be oxidized. Enzymatic dechlorination is key to the degradation of dichlorobenzene. Dichlorobenzene has fewer substituents. Compared with multi-substituted chlorobenzene compounds, the electron density on the benzene ring is relatively large, and there are more positions that can be attacked by molecules with oxidizing ability. It is easily oxidized and loses electrons, so it is also Easier to be biodegraded under aerobic conditions.
The degradation pathway basically follows the degradation mechanism of ring opening first and then degradation. Dichlorobenzene combines with molecular oxygen under the catalysis of the dioxygenase system (a multi-component enzyme system composed of flavoprotein, iron-sulfur protein and ferroprotein), and two oxygen atoms are inserted into the benzene ring. Oxidized to cyclic dichlorodiol, and then under the action of dehydrogenase, two hydrogen atoms are removed and converted into the corresponding dichloro-o-diphenol (catechol), which is then catalyzed by dioxygenase Convert to dichloromuconic acid to complete the ring opening process.
Dichloromuconic acid lactoneation occurs simultaneously with dechlorination, and the resulting product is gradually reduced to the point where dichlorobenzene is completely degraded or enters the tricarboxylic acid cycle. During the degradation process, dichloro-o-diphenol easily accumulates and causes greater toxicity to microorganisms. Some studies have found that adding yeast extract as an inducer during the reaction process, or gradually increasing the concentration of dichlorobenzene from a lower concentration for acclimation, can induce degrading microorganisms to produce o-diphenol degrading enzymes, completely degrading or partially converting dichlorobenzene. . For the three isomers of dichlorobenzene, different configurations result in different effective collision probabilities of being oxidized, which also results in differences in biodegradability.
For 1,2-dichlorobenzene, there are three adjacent positions with oxidizing ability on the benzene ring that can be attacked by two oxygen atoms; for 1,3-dichlorobenzene, these positions that may be attacked There are 2 adjacent positions; for 1,4-dichlorobenzene, due to the symmetrical distribution of substituent chlorine atoms, there is only one adjacent position that has oxidizing ability that can be attacked. As a result, the effective collision probability of being oxidized decreases successively. The biodegradability of 1,3-dichlorobenzene is lower than that of 1,2-dichlorobenzene, and the biodegradability of 1,4-dichlorobenzene is even lower than the first two.
Preparation[5]
The methods for obtaining dichlorobenzene are as follows:
1) Isolated from heavy by-products produced from chlorinated benzene, but the quantity is extremely limited;
2) Using ferric chloride as a catalyst, chlorinated benzene and chlorine as raw materials, further chlorination is carried out to obtain dichlorobenzene. Chlorobenzene is obtained by benzene chlorination, water washing, alkali washing, and distillation. This will The raw material cost of dichlorobenzene has increased significantly, and the chlorination of chlorobenzene to produce dichlorobenzene is also very slow, requiring a large investment in equipment;
3) Generally, a batch reactor is used to continuously pass chlorine gas into benzene containing ferric chloride to produce dichlorobenzene. This method is small in scale, has unstable product composition, and increases the amount of polychlorinated benzene by-products. Not conducive to post-processing;
4) Column continuous reactor, benzene, ferric chloride catalyst and chlorine are added continuously. Due to the lack of necessary stirring and the lack of suitable catalysts, the production capacity is low, the balance ratio is imbalanced, and the equipment investment is large. Not suitable for mass production. The key to producing dichlorobenzene is to select a suitable reactor, which should have the characteristics of high productivity, small size and simple structure. It is more important to choose a catalyst with high activity, high para-selectivity, cheap and non-toxic catalyst.
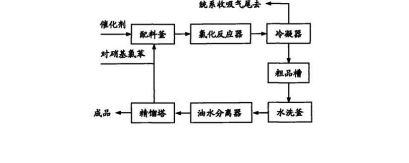
Some research has developed a method for the preparation of p-dichlorobenzene. The method is to continuously prepare p-nitrochlorobenzene in a continuous reactor. The continuous reactor is a reaction kettle with a built-in A reaction device connected to a tower with corrosion-resistant packing. The opening of the reaction kettle is directly connected to the inlet at the bottom of the tower body. The reaction kettle is provided with a feeding port and a vent, and the top of the tower is provided with a gas outlet.
The preparation of p-dichlorobenzene uses p-nitrochlorobenzene and continuously introduced chlorine as raw materials, and uses peroxide or azo compound as a catalyst, in a continuous reactor at 170~240°C. The denitrification reaction is carried out in the reactor to generate p-dichlorobenzene gas and tail gas nitroxyl chloride. Para-dichlorobenzene is taken out from the top outlet of the tower along with the tail gas nitroxyl chloride. Para-dichlorobenzene is condensed to obtain crude liquid p-dichlorobenzene. The crude p-dichlorobenzene is post-processed to obtain p-dichlorobenzene. In this way, continuous production of p-dichlorobenzene is achieved and the production scale is expanded.
Main reference materials
[1] Dictionary of Environmental Science
[2] Xu Yingming, Yuan Zhihua, Li Junxing, et al. Effects of dichlorobenzene stress on wheat seed germination and seedling growth [J]. Journal of Irrigation and Drainage, 2005, 24(4): 11-14.
[3] Ye Jing, Zhou Qi. Migration behavior and biodegradation of dichlorobenzene in soil[J]. Liaoning Urban and Rural Environmental Science and Technology, 2002, 22(5): 7-10.
[4] CN201410048269.7 Method for preparing chlorobenzene, p-dichlorobenzene and o-dichlorobenzene during chlorination of benzene
[5] CN200710156216.7 A preparation method of p-dichlorobenzene

 微信扫一扫打赏
微信扫一扫打赏

