Preparation【1】
Acylation of aromatic amines plays an important role in organic synthesis. As a protective measure, primary and secondary aromatic amines are often converted to their acetyl derivatives during synthesis to reduce the sensitivity of the aromatic amines to oxidants and prevent them from being destroyed by the reactive reagents. At the same time, after the amino group is acylated, its activation ability in the electrophilic substitution reaction (especially sulfation) is reduced, causing it to change from a strong Type 1 positioning group to a medium-strength Type 1 positioning group. The reaction changes from polyvalent substitution to useful monovalent substitution; due to the space effect of the acetyl group, para-substituted products are often selectively generated. In some cases, acylation can avoid unnecessary reactions between amino groups and other functional groups or reagents (such as RCOCl, SO2C1, HN02, etc.). In the final step of the synthesis, the amino group is readily regenerated by acid-base catalyzed hydrolysis of the amide. Aromatic amines can be acylated by acid chloride, acid anhydride or heating with glacial acetic acid. Glacial acetic acid reagent is easy to obtain and cheap, but it requires a long reaction time and is suitable for larger-scale preparation. Anhydrides are generally better acylating reagents than acid chlorides. Acylation with free amines and pure acetic anhydride is often accompanied by the formation of diethylamide [ArN(COCH3)2] as a by-product. But if in acetic acid. Acylation is carried out in a buffer solution of sodium acetate. Since the hydrolysis rate of the acid anhydride is much slower than the acylation rate, a high-purity product can be obtained. However, this method is not suitable for the acylation of nitroaniline and other weakly basic aromatic amines.
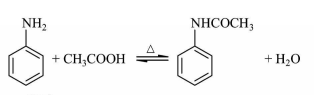
Acetanilide can be prepared by reacting aniline with reagents such as glacial acetic acid, acetic anhydride or acetyl chloride. Acetyl chloride is reactive in nature, difficult to preserve, and highly dangerous. Therefore, glacial acetic acid was used as the acylating reagent in this experiment.
The reaction equation is as follows:
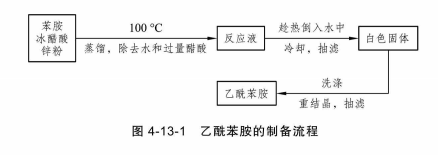
The experimental process is shown in the figure below:
Experimental steps
Add 2.0 mL of newly distilled aniline (2.04 g, 0.022 mol), 3.0 mL of glacial acetic acid (3.14 g, 0.052 mol) and A little zinc powder (about 0.02 g). Install a short thorn-shaped fractionating column with a thermometer on its upper end. The branch pipe is connected to the receiving bottle through the branch pipe connecting pipe. The outside of the receiving bottle is cooled with cold water. Place the mesh-bottomed flask on a heating mantle, heat and stir, and heat over low heat to keep the reactants at a slight boil for about 15 minutes. Then gradually increase the temperature. When the thermometer reading reaches about 100°C, liquid will flow out of the branch pipe. Heat it over low heat and keep the thermometer reading at 100-105°C for about 1 hour. The generated water and most of the acetic acid will be evaporated. A drop in temperature indicates that the reaction is complete. Pour the reactant into a beaker filled with 20 mL cold water while it is hot (preferably cooled with ice water). After cooling, filter the solid and crush the crystals. Wash the crystals with a small amount of cold water to remove residual residues. Acid, drain. The crude product can be recrystallized with water to obtain white flaky crystals, which are filtered, dried and weighed to calculate the yield. The melting point of acetanilide is 113~114℃ (literature value 114.3℃).
Notes
(1) Aniline that has been left for a long time is easily oxidized, so it is best to use freshly steamed aniline.
(2) The purpose of adding zinc powder is to prevent aniline from being oxidized during the reaction and producing colored impurities.
(3) After the reaction solution is cooled, the solid product immediately precipitates and sticks to the bottle wall, making it difficult to handle. Therefore, it should be poured into cold water with stirring while it is hot to remove excess acetic acid and unreacted aniline.
Recrystallization【2】
1. Single solvent method:
Take 0.5 g of crude acetanilide, place it in a 50 mL beaker, add a small amount of water, stir and heat until boiling, if it is still not completely dissolved, add a small amount of water until it is completely dissolved, then add 2 to 3 mL more Water (total volume is about 25 mL), cool slightly, add a little activated carbon, continue to heat and boil for 5 to 10 minutes, perform hot filtration under reduced pressure (you can preheat the Buchner funnel to a certain temperature first), and place the filtrate in a beaker , allow it to cool and crystallize. After the crystallization is complete, use a Buchner funnel to suction and filter, wash it with a small amount of water on the funnel, press it tightly and drain it, place the product in a watch glass to dry, weigh and measure its melting point. The melting point of acetanilide is 114°C.
When recrystallizing with water, oil beads often appear. This is because when the temperature is higher than 83°C, acetanilide that is not dissolved in water but has melted forms another liquid phase. At this time, just add a small amount of water Or continue to heat, this phenomenon will disappear.
2. Mixed solvent method:
Weigh 2.5 g of crude acetanilide, add it to a 50 mL round-bottomed flask, add about 15 ml of 15% ethanol solution, add 1 to 2 grains of zeolite, and install the reflux device. Open the condensed water, heat the water bath until the solvent boils, and keep refluxing for several minutes to observe whether the solid is completely dissolved. If there is insoluble solid or oily matter, add 3 mL of solvent from the top of the condenser tube, then heat and reflux for a few minutes, and add solvent one after another until the solid or oily matter is completely dissolved to obtain a hot saturated solution, and then add an excess of 5 mL. ~10 mL solvent. Remove the water bath, and after the solution cools slightly, add activated carbon, continue heating in the water bath, and boil under reflux for 10 to 15 minutes. During the reflux period, preheat the Buchner funnel and filter flask by boiling them in a hot water bath. Install the preheated suction filtration device, filter the hot solution while it is hot, and pour the filtrate into a clean hot beaker as soon as possible. Let the filtrate slowly cool to room temperature, crystals will precipitate, and then proceed.�Filter with suction, wash the crystals with a small amount of water, and drain to obtain white flaky crystals. The product is dried and weighed, and the recrystallization yield is calculated.
Pure acetanilide is colorless scaly crystals. The purity of the product can be identified by measuring the melting point.
References
[1] Liu Wei, editor-in-chief Gou Gaozhang, Comprehensive Chemistry Experiment 1, Southwest Jiaotong University Press, 2016.08, page 94
[2] Editor-in-chief Kai Cheng; Yang Ming, deputy editor-in-chief Wei Benmei, Chemistry Experiment Tutorial, Huazhong University of Science and Technology Press, 2014.02, page 111

 微信扫一扫打赏
微信扫一扫打赏

