Background and overview[1]
O-phenylphenol (OPP for short) is an organic chemical product with a wide range of uses. It is widely used in sterilization and antisepsis, printing and dyeing auxiliaries and surfactants, and stabilizers and inhibitors for the synthesis of new plastics, resins and polymer materials. Fuel and other fields. OPP and its sodium salt have broad-spectrum sterilization and mildew removal capabilities, and are low-toxic and odorless. They are good preservatives and can be used to prevent mildew and preserve fruits and vegetables. They are especially suitable for citrus to prevent mildew and can also be used to treat lemons. , pineapples, pears, peaches, tomatoes, cucumbers, etc., can minimize rot. OPP and its sodium salt can also be used in cosmetics, wood, leather, fiber and paper as antiseptic and bactericidal agents. OPP and its water-soluble sodium salt can be used as dye carriers for polyester fibers, and can also be used as carriers for carrier dyeing of polyester fibers, polyester fibers, etc. By replacing phenol with OPP, a new phenolic resin can be synthesized. This new resin has high thermal stability and low water absorption, and can be used to prepare paints with excellent stability to water and alkali.

Preparation[1]
Regarding the synthesis of OPP, the main methods are: the two-step dehydration-dehydrogenation method of phenol reported in US4035428 or the dehydrogenation of diphenyl ether to generate the intermediate dibenzofuran, and then hydrogenation of the intermediate to synthesize OPP , the reaction yield is 45~55%; DE1930341 reports the synthesis of OPP by chlorobenzene hydrolysis, the conversion rate of chlorobenzene is 95%, and the OPP in the product is only 11%; US4000203 reports the synthesis of OPP by ring opening of dibenzofuran, the product The dibenzofuran content is 75%, and the OPP is 3%; JP55047636 reports that cyclohexanone and phenol are reacted to synthesize OPP. In all results, the conversion rate of cyclohexanone is not higher than 75%, and the selectivity of OPP is not higher than 45%; JP50108244 reports that phenol can be directly synthesized into OPP, and its conversion rate and selectivity are both 20%. The above synthesis methods all have the shortcomings of low conversion rate and selectivity; some methods use chlorine-containing phenolic substances as raw materials, which cause major pollution and are unfriendly to the environment; some methods have too high raw material costs, resulting in high industrialization costs. The synthesis method currently widely studied is to use cyclohexanone as raw material, first dehydrate and dimerize into cyclohexanone dimer, and then catalytically dehydrogenate to generate OPP. The reaction formula is as follows:
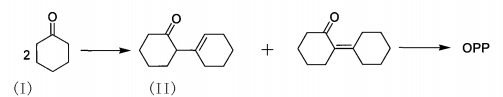
Among them, 2-(1-cyclohexenyl)cyclohexanone (I) and 2-cyclohexylalkylenecyclohexanone (II) are isomers. There is no need to separate them during synthesis and can be directly Dehydrogenation occurs under the action of a catalyst to form OPP. This method has convenient sources of raw materials, low cost, generates water and hydrogen at the same time, has less pollution, and is suitable for industrial production.
At present, most of the catalytic dehydrogenation links reported in this synthesis method adopt the gas phase method, that is, using noble metals such as Pt, Pd, Ru, Ir as the main components, and the carrier is mostly Al2O 3, modify the carrier and carry out gas-solid heterogeneous reaction in a fixed bed reactor.
For example, JP56-053632 describes the use of Pt/γ-Al2O3 or Pt/SiO2– Al2O3 is used as a catalyst, and the dimer is reacted with the catalyst in the gas phase at 250~550℃.After contact synthesis of OPP, after 3000 hours of use, the dimer conversion rate was 95% and the OPP selectivity was 92.1%. Wei Yan’an (“Applied Chemistry”, 2004, vol. 21(8): p659~861) et al. used carbon-coated γ- Al2O3 as the carrier. Using 0.5% Pt and 1% K2O as catalyst, after 100 hours of use, the dimer conversion rate was 95%, and the OPP selectivity reached 85.1%. JP49‑041348 describes that using 1% Pd‑16% KOH/γ‑ Al2O3 catalyst at 350℃, After reacting for 100 hours at a hydrogen flow rate of 3L/h and a dimer LHSV of 0.3h-1, the dimer conversion rate was 100% and the OPP selectivity was 83%. DE3523205 describes the use of a Ru/γ-Al2O3 catalyst, which also contains Cr, Mn, alkali metals, and sulfur-containing compounds. The catalyst is used to synthesize OPP by a gas phase method at 330 to 350°C. After reacting for 48 hours under the condition of polymer 0.2g/(mlCat·h), the OPP content in the product was 88%. US4080390 describes using γ‑ Al2O3 as a carrier, loading 0.1% Pt, 5% KOH and 0.2% Ir. The dimer was catalyzed for dehydrogenation at 350°C, the dimer flow rate was 9 mL/h, and hydrogen was used as the carrier gas. Within the first 150 hours of use of the catalyst, the dimer conversion rate was 93%, and the OPP selectivity was as high as 95%.
In addition, other substances are used as catalysts, such as JP55-047636 one or more metals or their oxides Zn, In, V, Zr, Cr, Mo, W, Ti , Ni, Nb, Pb, Ta as catalysts, or a mixture of one or more of the above metals or their oxides and one or more of Pd, Pb, Rh, Ir, Ru, and Cu as catalysts to catalyze dimer removal. The conversion rate of hydrogen and raw materials is about 95%, but the OPP selectivity does not exceed 25%.
DE2049809 describes the use of carbonates or hydroxides containing 40 to 60% Ni, 6.8 to 17.1% Cr, 1.6 to 8% Al, and 0.05 to 1% Cu, and adding alkali metal sulfates or carbonic acids. salt, pressed into tablets to make a catalyst, and hydrogen is used to reduce the Ni in it. When a catalyst containing 52.4% Ni is used for dehydrogenation reaction in gas-solid form, the dimer conversion rate is 89% in 11h, and the OPP selectivity is 94% in 280h. Afterwards, the conversion rate dropped to 65% and the selectivity dropped to 85%. The above gas phase method for synthesizing OPP generally has the problem of poor catalyst stability. The activity and selectivity of the catalyst begin to decrease after a short period of use.
CN200910098009 overcomes the shortcomings of the existing technology and provides a method for synthesizing o-phenylphenol.
Method for synthesizing o-phenylphenol: Add the Pt/C catalyst modified with alkali metal or alkaline earth metal carbonate and cyclohexanone dimer into the reaction kettle, fill it with nitrogen and replace it 2 to 3 times, and stir Heating to 320~400℃, the reaction pressure is 0.6~2MPa, when the content of the intermediate o-cyclohexylphenol in the reaction solution is less than 2%, the reaction is stopped, cooled to 60~70℃, filtered, and o-phenylphenol containing of filtrate. The core of the present invention is to modify the activated carbon carrier with alkali metal or alkaline earth metal carbonate, thereby inhibiting the promotion effect of the acidic center on the activated carbon on the decomposition of the reaction raw material cyclohexanone dimer, and at the same time providing for the generation of o-phenylphenol. Appropriate alkaline conditions, thereby greatly improving the selectivity of the catalyst. In addition, the process unit of the present invention has high catalyst production efficiency, convenient catalyst recovery, and is beneficial to industrialization.
Apply [2]
The use of o-phenylphenol in high concentrations is suitable for inhibiting the growth of fungi (fungistatic effect) or even for killing fungi (fungicidal effect). CN200580026288 discloses the use of o-phenylphenol and/or its derivatives for inhibiting asexual reproduction of fungi, and also relates to filter media, adhesives, building materials, building accessories, and fabrics containing o-phenylphenol and/or its derivatives. , fur, paper, leather or leather products, as well as detergents, cleaning agents, rinse aids, hand detergents, manual dishwashing detergents, automatic dishwashing detergents, and for finishing building materials, building accessories, fabrics, furs, paper , reagents for leather or leather products, etc.
Main reference materials
[1] CN200910098009.X A method for synthesizing o-phenylphenol
[2] CN200580026288.9 O-phenylphenol and/or its derivatives are used to inhibit asexual reproduction of fungi

 微信扫一扫打赏
微信扫一扫打赏

