Background and overview[1]
Benzophenone hydrazone is an important raw material for the synthesis of photosensitive materials, spices and medicines, and an intermediate for ultraviolet absorbers, organic pigments and pesticides. In the pharmaceutical industry, in addition to being used in the production of dipipridine, diphenhydramine, etc., benzophenone hydrazone is also an important precursor for the synthesis of cardiovascular drugs cibendazoline. In the synthesis of drugs such as β-lactam inhibitor tazobactam, it is also widely used as the carboxyl protecting group of 6-APA and 7-APA. Benzophenone hydrazone can also be used to prepare diphenyldiazomethane and related compounds. Diphenyldiazomethane is a valuable intermediate in the peptide synthesis process. At the same time, benzophenone hydrazone is also the main raw material for the preparation of indole. Indole can be used to synthesize a variety of drugs, especially the raw material for tryptophan, one of the indispensable amino acids in the human body. Indole is also a component of many spices. .

Preparation[1]
Currently, there are three reports on the synthesis of benzophenone hydrazone in the literature.
Method 1: Prepared by refluxing anhydrous hydrazine and benzophenone in anhydrous ethanol for 5 to 7 hours. However, the anhydrous hydrazine used in this method is a highly self-igniting and self-exploding reagent, which is extremely unsafe and is not available on the market. The preparation of anhydrous hydrazine is to use hydrazine hydrate with a volume concentration of 80 to 85% and potassium hydroxide to reflux and dehydrate and then distill to obtain anhydrous hydrazine with a water content of ≤2.0%. This method not only consumes a large amount of potassium hydroxide, but also the distillation device must be strictly sealed, otherwise the anhydrous hydrazine vapor escaping during the distillation process will often spontaneously ignite, which is extremely dangerous. Therefore, this method cannot be industrialized.
Method 2: Use ethylene glycol as the solvent, replace anhydrous hydrazine with hydrazine hydrate with a volume concentration of 80%, and react with benzophenone for 3 hours to obtain benzophenone hydrazone.
Method 3: Using ethanol as the solvent, benzophenone and 85% hydrazine hydrate are refluxed for 9 hours in the presence of hydrochloric acid to obtain benzophenone hydrazone. The latter two methods both use 80 to 85% hydrazine hydrate as raw material. In short, the hydrazine hydrate concentration requirements used in these previous methods are relatively high. Even if 50% hydrazine hydrate is used, the reaction cannot proceed smoothly. This makes these methods more expensive, and there is a large excess of hydrazine hydrate, which causes greater environmental pollution.
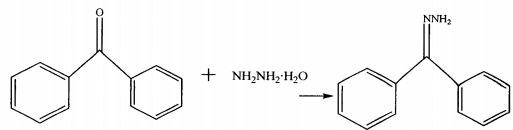
CN200610051887 provides a method for synthesizing benzophenone hydrazone with simple operation, mild reaction conditions, high yield, easily available raw materials, low cost, and little environmental pollution, which can use lower concentration of hydrazine hydrate as raw material. The technical solution adopted by the present invention is as follows: a method for synthesizing benzophenone hydrazone. Benzophenone and hydrazine hydrate are reacted in an ionic liquid at 80-140°C for 0.5-6 hours. The reaction product is post-processed to obtain the dibenzophenone hydrazone as shown. Benzophenone hydrazone, the reaction formula is as follows:
After the present invention uses ionic liquid as the reaction solvent, it can react with low-concentration hydrazine hydrate and benzophenone. The beneficial effects are mainly reflected in: (1) The use of ionic liquid is not easy to volatilize, is not flammable and explosive, and is safe. Good, and has good solubility in both organic and inorganic substances. The reaction is carried out under homogeneous conditions, which is easy to operate and handle.The product yield is high. (2) Ionic liquids can be recycled and have little impact on the environment. (3) Replacing high-concentration hydrazine hydrate or anhydrous hydrazine with low-concentration hydrazine hydrate shortens the reaction time, increases product yield, reduces process costs, is more economical, safer, and environmentally friendly, and raw materials are easily available, which is conducive to industrialization. Production.
Apply [2]
Benzophenone hydrazone can be used to synthesize diphenyldiazomethane. Diphenyldiazomethane is widely used as a carboxyl protecting reagent in drug synthesis. It is also used in the analysis of carboxylic acid mixtures and the synthesis of organic raw materials. In addition, it is a useful alkylating agent. At present, the main synthesis method of diphenyldiazomethane is to oxidize benzophenone hydrazone. In patents US 4092306 and EP0177248, the oxidants used mainly include HgO, MnO2, NiO, and hexafluoro Acetone, H2O2, Pb(C2H3O2 )4, peracetic acid, chloramine-T, methanesulfinyl chloride method, etc. This oxidation method is simple to operate and does not require harsh conditions. However, the currently used oxidants have shortcomings such as large usage, serious pollution, and low product purity.
CN200710070387.8 provides a safe synthesis method that is industrially feasible, has high yield, and can prepare high-purity diphenyldiazomethane. In order to achieve the purpose of the invention, the present invention adopts the following technical solution: a synthesis method of diphenyldiazomethane, which uses benzophenone hydrazone as a raw material, and the reaction conditions are organic solvents that are immiscible with water. In the presence of hydrogen peroxide as an oxidant and iodine as a catalyst, in the presence of an organic amine and a phase transfer catalyst, the oxidation reaction is carried out at 10 to 50° C. for 1 to 8 hours, and the reaction product is post-processed to obtain diphenyldiazomethane, as described The amount ratio of benzophenone hydrazone, organic amine, iodine, phase transfer catalyst: hydrogen peroxide is 1:1~4:2×10-4~6×10-4: 0.5×10-2~2.5×10-2: 4~20, the phase transfer catalyst is tetrabutylammonium bromide. The synthesis method of diphenyldiazomethane of the present invention includes the following steps: adding benzophenone hydrazone, organic amine, catalyst iodine, and phase transfer catalyst tetrabutylammonium bromide to an organic solvent immiscible with water. , at a temperature of 10 to 50°C, slowly add hydrogen peroxide to perform an oxidation reaction. After the reaction is completed, the reaction product is post-processed to obtain high-purity diphenyldiazomethane. Usually it takes 0.5 to 3 hours to slowly add hydrogen peroxide, and the oxidation reaction takes 1 to 8 hours.
Main reference materials
[1] CN200610051887.2 A kind of synthesis method of benzophenone hydrazone
[2]CN200710070387.8 A kind of synthesis method of diphenyldiazomethane

 微信扫一扫打赏
微信扫一扫打赏

