Background and overview[1]
2-Iodobenzoic acid is also known as o-iodobenzoic acid. o-iodobenzoic acid and its derivatives are important pharmaceutical and chemical raw materials and necessary for the synthesis of various high-iodine reagents.
Preparation[1]
The method of synthesizing o-iodobenzoic acid and its derivatives recorded in domestic and foreign literature is to use anthranilic acid as raw material and obtain the product through a two-step reaction of diazotization and iodination.
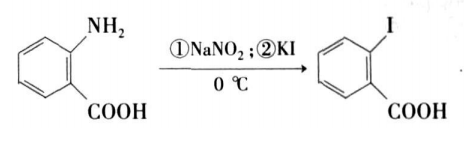
Yang Yiheng et al. designed and implemented another synthesis route, that is, directly using cheap phthalic anhydride or phthalic anhydride derivatives as raw materials, o-iodobenzoic acid and its derivatives can be synthesized through the three-step reaction of hydrolysis, mercury substitution and iodo substitution. The structure of the product was confirmed by melting point, 1HNMR and IR characterization. The synthesis route is as follows:
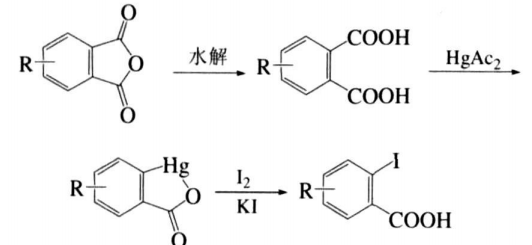
Compared with the traditional method, the raw materials used in this method are widely sourced and low in price. The reaction process has good operability and strong versatility, and is suitable for the preparation of o-iodobenzoic acid and its various derivatives. The details are as follows:
Synthesis of o-iodobenzoic acid:
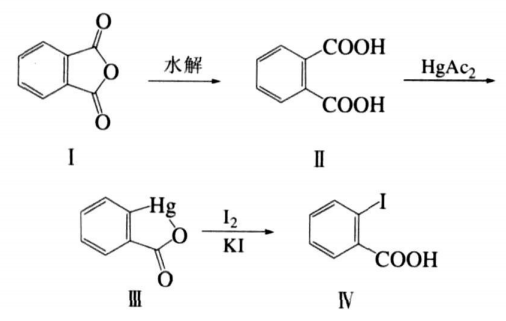
1 Mercury substitution reaction
Add 7.4 g (0.05 mol) phthalic anhydride (Ⅰ) into 300 mL (NaOH )=0.5mol/L aqueous solution, heat and reflux until the solid is completely dissolved, then add an appropriate amount of acetic acid to neutralize the excess alkali. The solution is weakly acidic and II is precipitated. In addition, 11.0 g (0.055mol) mercuric oxide was added to the acetic acid solution (acetic acid 25mL, water 50mL), heated to reflux until the solid was completely dissolved, to obtain a mercury acetate solution, and added to the suspension of the above II, plus Acidify with 10 mL of acetic acid, reflux for 24 hours, and then cool and filter to obtain 15.6 g of white solid III, which is washed with water and dried. It is the decarboxylated mercury product III of phthalic acid, with a melting point of >350°C and a yield of 98%.
2 Iodination reaction
Add the above white solid III 15.6 g (0.048mol) and 13 g (0.050mol) iodine to 200mL (0.50mol/L) KI aqueous solution, and heat it back to�15h. Cool the reactant, filter out the solid insoluble matter, add 20 mL (0.5 mol/L) sodium thiosulfate aqueous solution to the filtrate, stir for a moment, then acidify with hydrochloric acid, a white solid will precipitate, filter out the white solid, wash with water, and dry , recrystallized with ethanol to obtain 10.3 g of o-iodobenzoic acid IV, with a total yield of 82.5%. The melting point was measured to be 164°C (literature value 163°C). I R (KB r ) ,ν/c m-11 690~1677;1HNMR (CDCl3, 300MHz): δ=7. 98(d, 1H), 7.70 (d, 1H), 7.48(t, 1H), 7.23(t, 1H).
Apply [2-3]
1. Synthesis of secondary trifluoromethyl propargyl alcohol
Trifluoromethyl propargyl alcohols are an important class of fluorine-containing functional building blocks that can be further derivatized to synthesize diversified fluorine-containing functional organic molecules. Therefore, it is of great significance to develop synthetic methods for the synthesis of trifluoromethyl propargyl alcohol derivatives.
CN201710140153 provides a synthesis method for preparing secondary trifluoromethyl propargyl alcohol using 2-iodobenzoic acid. The method of the present invention can effectively synthesize secondary trifluoromethyl propargyl alcohol. Including the following steps:
1, 2-iodobenzoic acid and sodium periodate react in acetic acid aqueous solution to form 1-(hydroxy)-1,2-phenyliodonyl-3(1H)-one;
2. Dissolve trimethylsilyl acetylene in the solvent, react with triisopropylsilyl chloride under the action of n-butyllithium to generate trimethylsilyl (triisopropylsilyl) acetylene;
3. Dissolve the 1-(hydroxy)-1,2-pheniodoyl-3(1H)-one prepared in step 1 in the solvent, and mix it with trimethylsilyl trifluoromethanesulfonate. The trimethylsilyl (triisopropylsilyl) acetylene prepared in step 2 is reacted, and finally 1-[(triisopropylsilyl)ethynyl]-1,2-phenyliodoyl- is synthesized under the action of pyridine. 3(1H)-ketone;
4. Combine 1-[(triisopropylsilyl)ethynyl]-1,2-pheniodoyl-3(1H)-one prepared in step 3, trifluoroethylamine hydrochloride and nitrous acid Sodium is dissolved in the solvent and reacts under the action of copper catalyst to form the trifluoromethyl propargyl alcohol derivative 1,1,1-trifluoro-4-triisopropylsilyl-3-butyne-2-o- Iodomethylbenzoate;
5. Prepare the trifluoromethyl propargyl alcohol derivative 1,1,1-trifluoro-4-triisopropylsilyl-3-butyne-2-o-iodobenzoic acid methyl The ester is added to the solvent and hydrolyzed under alkaline conditions to obtain 1,1,1-trifluoro-4-triisopropylsilyl-3-butyn-2-ol.
Compared with the existing technology, the beneficial effects of the present invention are reflected in:
1. The secondary trifluoromethyl propargyl alcohol method provided by the present invention synthesizes 1-[(triisopropylsilyl)ethynyl]-1,2-phenyliodoyl-3( 1H)-ketone, and finally the secondary trifluoromethyl propargyl alcohol can be obtained through hydrolysis;
2. The method of secondary trifluoromethyl propargyl alcohol obtained by the present invention is low in toxicity and does not produce other polluting impurities, and the by-product o-iodobenzoate can be recycled;
3. The secondary trifluoromethyl propargyl alcohol synthesized by the present invention has many important applications in the field of drug synthesis due to its good chemical stability, hydrophobicity and metabolic stability.
2. Used in the synthesis of trifluoromethyl aromatic compounds
Limited by effective synthesis methods, the wide application of trifluoromethyl compounds (especially trifluoromethyl aromatic and aromatic heterocyclic compounds) has limitations. CN201611185974.7 provides a method for preparing fluoromethyl aromatic compounds using 2-iodobenzoic acid. A method for synthesizing trifluoromethyl aromatic compounds includes the following steps: 1) Synthesizing Tony’s reagent α using sodium periodate, 2-iodobenzoic acid, acetic anhydride, cesium fluoride and Ruppert reagent as raw materials. 2) Dissolve the aromatic amine and hydrochloric acid in DCE, stir for 5-10 minutes, add tert-butyl nitrite at low temperature, stir for 30-60 minutes, and then add copper tetrafluoroborate, Tony’s reagent α, and hydrogen carbonate. Sodium, reaction is slightly hot. After the reaction is completed, filter, wash, dry and filter in sequence, and purify through column chromatography to obtain the target product.
Main reference materials
[1] Yang Yiheng, Zhang Shuangquan, Tang Chenghe, Song Guoqiang. Synthesis of o-iodobenzoic acid and its derivatives [J]. Fine Chemicals, 2007(08):813-815+819.
[2] CN201710140153.X A synthesis method of secondary trifluoromethyl propargyl alcohol
[3]CN201611185974.7 A synthesis method of trifluoromethyl aromatic compounds

 微信扫一扫打赏
微信扫一扫打赏

