Overview
Ethylbenzene, phenylethane, is called ethylbenzene in foreign languages, and its molecular formula is C8H10 (C6H5 CH2CH3), molecular weight is 106.17, colorless liquid, aromatic odor, insoluble in water, miscible in most organic compounds such as ethanol and ether. Solvent, relatively stable in nature, used in organic synthesis and as a solvent. It is highly irritating to the skin and mucous membranes, and has an anesthetic effect at high concentrations.
Synthesis process[3]
Method 1: AlCl3 method. The AlCl3 method uses a typical Friedel-C rafts process, using AlCl3 complex. catalyst. The by-products of the reaction are mainly diethylbenzene and polyethylbenzene, and there are traditional AlCl3 method and improved AlCl3 method.
In the traditional AlCl3 method[1], the reactants and catalyst in the reactor form three phases, liquid aromatics, gaseous ethylene and liquid catalyst complex. The catalyst complex is red and is immiscible with liquid aromatics. During the reaction, ethylene bubbles into the reactor containing two liquid phases, causing them to disperse and mix. The molar ratio of ethylene to benzene is 0.3 to 0.35, and the reaction is carried out below 130°C and under normal pressure. The process flow is shown in Figure 1.
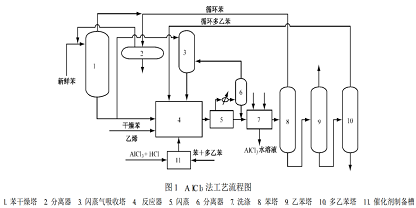
The conversion rate of ethylene is close to 100%, the yield of ethylbenzene is high, and the amount of recycled benzene and ethylbenzene is small; the alkylation reaction of benzene and ethylene and the transalkylation reaction of polyethylbenzene can be performed on the same station completed in the reactor. The disadvantages are that the reaction medium is highly corrosive, the equipment cost and maintenance cost are high, and the organic phase of the reaction product is washed with water and alkali to produce a large amount of wastewater containing aluminum hydroxide slurry, plus waste catalyst, causing serious environmental pollution.
Improved AlCl3 method[2], due to the traditional AlCl3 method, there are serious pollution, corrosion and two problems in the reactor. Liquid phase and other issues, in 1974 M on san to/L umm u s company proposed an improved AlCl3 method, which greatly reduced the amount of AlCl3 catalyst (only the traditional 1/3 of the method), thus reducing the amount of waste catalyst treatment, and the feed ethylene concentration range can be 15% to 100%. By controlling the feed of ethylene, the amount of AlCl3 catalyst is reduced to within the solubility range, so that the reaction can be carried out in a uniform liquid phase and the yield of ethylbenzene is increased.
The reaction temperature is 160~180℃, the pressure is 0.6~0.8MPa, and the molar ratio of ethylene to benzene is 0.8. When dilute ethylene is used as the raw material, H2S, O2, CO2 and H2 will appear in the feed gas. >O needs to be purified to a mass fraction of approximately 5×10-6. Since this method has an obvious effect in reducing costs, many traditional AlCl3 method devices have been transformed and expanded using the Mon Santo/Lummu s method. However, this method only alleviates the problems of equipment corrosion and environmental pollution, but does not fundamentally solve them.
The process flow of the improved AlCl3 method is similar to the traditional AlCl3 method. The difference is that the alkylation and transalkylation reactions are carried out in two reactors. conduct. In the improved AlCl3 method, only dry benzene (instead of benzene and recycled polyethylbenzene), ethylene, and catalyst are continuously fed into the alkylation reaction, and the alkylation reaction generates After the material is mixed with the recycled polyethylbenzene from the distillation tower, a transalkylation reaction is performed in another reactor.
Method 2 A lkar method[3], the A lkar method was developed by UOP in 1958 and industrialized in 1960. It uses a load in A l2O< BF3 on sub>3 is the catalyst. Ethylene with a concentration as low as 8% to 10% (mass fraction) can be used as raw material for alkylation reaction, so treated FCC dry gas or coke oven exhaust gas can be used as raw material. The reaction is carried out at 100~150℃ and 2.5~3.5MPa, and the molar ratio of ethylene and benzene is controlled between 0.15~0.2. The transalkylation reaction is carried out in another reactor at a temperature of 180 to 230°C. The materials from the two reactors are combined and entered into the purification system. The purity of ethylbenzene in the finished product can reach 99.9%.
The main advantages of this method are high catalyst activity, long life, good ethylbenzene selectivity, no corrosion and no pollution, short process, low energy consumption, and can be used for the comprehensive utilization of low-concentration ethylene. The disadvantage is that the catalyst preparation conditions are harsh and expensive.>Polyethylbenzene–>C8aromatic hydrocarbons
Downstream product propylene oxide–>acetophenone–>mesitylene–>p-nitroacetophenone–>food coloring brilliant blue–>1-(2-isocyanoethyl)- 4-Methylpiperazine–>(+)-1-phenyl-2-(p-methylphenyl)ethylamine–>Iopanoic acid–>Α-butyl-Α-phenyl-1H- Imidazole-1-propionitrile–>2-(tributyltin)pyrimidine–>N-tert-butyl-N’-(4-ethylbenzoyl)-3,5-dimethylbenzoylhydrazide– >p-Ethylbenzoic acid–>4-octylbenzaldehyde–>4-hydroxyphenylethyl methyl ether–>4-ethylphenol–>procainamide hydrochloride–>methylbenzyl alcohol –>Acid Red B–>2-Ethylanthraquinone–>2-Acetylbenzimidazole–>1,4-diethylbenzene
Main purposes
1. Used as raw material for styrene, also used in pharmaceuticals and other organic synthesis
2. Ethylbenzene is mainly used to produce styrene, and then to produce styrene homopolymers and copolymers with styrene as the main component (ABS, AS, etc.). Ethylbenzene is used in a small amount in the organic synthesis industry, such as the production of intermediates such as acetophenone, ethyl anthraquinone, p-nitroacetophenone, and methyl phenyl ketone. In medicine, it is used as an intermediate of synthromycin and chloramphenicol. Also used in spices.
3. Used as chromatographic standard materials and solvents, and also used in organic synthesis
Health Hazards
Routes of invasion: inhalation, ingestion.
Health hazards: This product is highly irritating to the skin and mucous membranes, and has an anesthetic effect at high concentrations.
Acute poisoning: Mild poisoning has symptoms of dizziness, headache, nausea, vomiting, staggering gait, mild disturbance of consciousness, and eye and upper respiratory tract irritation. Severe cases may cause coma, convulsions, drop in blood pressure and respiratory and circulatory failure. Liver damage may occur. Direct inhalation of this liquid can cause chemical pneumonitis and pulmonary edema.
Chronic poisoning: eye and upper respiratory tract irritation symptoms, neurasthenic syndrome. The skin appears sticky, cracked, and peeling.
Accidental leakage treatment
Quickly evacuate personnel in the leaked contaminated area to a safe area, isolate them, and strictly restrict access. Cut off the fire source. Quickly use sand and mud to block the spread of ethylbenzene spilled on the ground. Build dams to cut off the flow of contaminated water bodies, or use fences to limit the spread of ethylbenzene on water surfaces. Wear a gas mask and gloves, collect the leaked liquid in an appropriate container and seal it, use sand or other inert materials to absorb the leaked liquid, and transfer it to a safe area. When ethylbenzene is spilled into the soil, immediately collect the contaminated soil and move it to a safe area. Strengthen ventilation in contaminated areas, evaporate residual liquid, and eliminate ethylbenzene vapor.
Protective measures
Respiratory system protection: When the concentration in the air exceeds the standard, a self-priming filter gas mask (half mask) should be worn. During emergency rescue or evacuation, air respirators or oxygen respirators should be worn.
Eye protection: Wear chemical safety glasses.
Body protection: Wear anti-virus and penetration overalls.
Hand protection: Wear latex gloves.
Others: Smoking, eating and drinking are prohibited at the work site. After work, take a shower and change clothes. Maintain good hygiene habits.
First aid measures
Skin contact: Take off contaminated clothing and wash skin thoroughly with soap and water.
Eye contact: Lift eyelids and rinse with running water or saline. Seek medical attention.
Inhalation: Leave the scene quickly to fresh air. Keep your airway open. If breathing is difficult, give oxygen. If breathing stops, perform artificial respiration immediately. Seek medical attention.
Ingestion: Drink plenty of warm water, induce vomiting, and seek medical attention.
Fire-fighting method: Spray water to keep the fire container cool. Move containers away from the fire to an open area if possible. Containers in a fire scene must be evacuated immediately if they change color or if there is sound from the safety pressure relief device. Fire extinguishing media: foam, dry powder, carbon dioxide, sand. Water is ineffective in extinguishing the fire.
References
[1] H ervert G L , Grove D , L inn C B. U S: 2 939 890,1960-06-07.
[2] App legathf F, D u P L , M acfarlane, et al. U S: 3 848 012, 1974.
[3] Yang Liying, Wang Zhiliang, Zhang Jirui, et al. Research progress on ethylbenzene synthesis and production technology and technology [J]. Chemistry World, 2001, 42(10):545-549.

 微信扫一扫打赏
微信扫一扫打赏

