Background and overview[1][2]
O-methylbenzonitrile and its derivatives are important organic synthesis intermediates. At present, the main methods for preparing o-methylbenzonitrile and its derivatives are: 1) Nitrilation reaction between CuCN and halogenated aromatic hydrocarbons to prepare aromatic nitrile compounds. The advantage of this nitrification method is that it does not require catalysts and ligands, and can be reacted at high temperature in a suitable solvent. This method is still used in the industrial production of aromatic nitrile-based compounds. The disadvantage of this method is that the reaction conditions are harsh, require high temperatures (150~250°C), and require high equipment. 2) Use CuI as the catalyst, DMDEA as the ligand, and toluene as the reaction medium to catalyze brominated aromatic hydrocarbons to prepare aromatic nitrile compounds. After 24 hours of reaction, the yield of aromatic nitrile compounds is 70% to 98%.
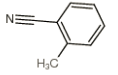
Structure
Preparation[1]
A method for preparing high-purity o-methylbenzonitrile and its derivatives, which at least includes the following steps:
1) Add the inorganic base and palladium catalyst to the organic solvent, stir, add the ligand, raise the temperature to 100~140°C, add o-methyl bromobenzene and its derivatives, and then add the nitrile reagent, inert Under gas protection, react for 10 to 15 hours; add an inorganic base accounting for 62% of the weight of the reaction substrate and a palladium catalyst accounting for 0.05% of the weight of the reaction substrate into an anhydrous organic solvent with a volume of 2.28 times the weight of the substrate, and stir thoroughly. Then add the reaction substrate and a nitrile reagent accounting for 0.16 to 0.25 times the mole amount of the substrate, and react for 10 to 15 hours under the protection of an inert gas. Check that the reaction is complete, quench the reaction with distilled water, extract with n-hexane, separate the organic layer, wash with saturated NaCl aqueous solution, dry the organic phase with anhydrous sodium sulfate or magnesium sulfate for 12 hours, and evaporate the solvent under reduced pressure to obtain the crude product. Inorganic bases include sodium carbonate, potassium carbonate, sodium bicarbonate, etc., preferably sodium carbonate. Palladium catalysts include palladium acetate, palladium triphenylphosphine, palladium chloride, etc., preferably palladium acetate. Anhydrous organic solvents include N,N-dimethylacetamide, N,N-dimethylformamide, N-methylpyrrolidone, dimethyl sulfoxide, etc., preferably N-methylpyrrolidone. Nitrilation reagents include potassium ferrocyanide, potassium ferrocyanide·3H2O, etc., preferably potassium ferrocyanide. The molar ratio of o-methyl bromobenzene and its derivatives to the nitrile reagent is 1:0.16~0.25, preferably 1:0.19.
2) Purify the crude product obtained in 1) to obtain the pure product. Step 2) mainly involves the purification process of the crude product. Due to differences in the physical properties of the obtained o-methylbenzonitrile and its derivatives, for example, 4-fluoro-2-methylbenzonitrile is a solid at normal temperature and pressure, while o-methylbenzonitrile is a liquid at normal temperature and pressure. . Therefore, different purification methods are required for different traits.
There are mainly two ways:
1) Recrystallization: Put the crude product into a three-necked flask, add ether solvent, raise the temperature to about 40°C, and stir until completely dissolved. Slowly cool to 20°C, stir at this temperature for 2 hours, then cool to 0°C, stir, crystallize, filter, and dry to obtain pure product with a purity of 99.83%.
2) Distillation: Put the crude product in a single-neck bottle, set up a distillation device, and collect the fractions at -0.006MPa and 100°C to obtain pure product with a purity of 99.75%. The ether reagent includes one or two of petroleum ether and methyl tert-butyl ether, and preferably the two are used in a certain ratio to recrystallize the corresponding product. The amount of recrystallization solvent is 12 to 2.8 times the volume of the crude product, preferably 2.5 times.
Apply[2-3]
O-methylbenzonitrile and its derivatives are important organic synthesis intermediates. They are widely used in the preparation of pesticides, medicines, dyes and other fine chemical products. In recent years, with the popularity of polyester fluorescent whitening agents in China It is widely used and the demand for o-methylbenzonitrile and its derivatives as its important raw materials is also increasing. Examples of its application are as follows:
1. Synthesis of multi-substituted pyridine derivatives and bipyridine derivatives from nitriles. Pyridine derivatives are an important class of organic compounds and are the basic components of many natural medicines, pesticides, dyes, vitamins and various alkaloids. Pyridine derivatives and bipyridine derivatives play an important role in chemical biology and many interdisciplinary fields. For example, as a compound for metal-organic compounds��, playing an important role in coordination chemistry, polymer materials science and metal-organic chemistry.
Using nitriles as direct raw materials, cyclization with 1,4-diiodo-1,3-butadiene derivatives, which is easily available, can synthesize multi-substituted pyridine derivatives and bipyridine derivatives with high yield and selectivity. things. The synthesis method includes first reacting 1,4-diiodo-1,3-butadiene derivatives dissolved in diethyl ether or tetrahydrofuran solvent with n-butyllithium or tert-butyllithium at a temperature of -78°C to -50°C. , then add nitrile and hexamethylphosphoramide. Warm the reaction solution to 20°C-28°C, quench the reaction with saturated NaHCO3 aqueous solution after 0.5-1.5 hours, and then extract, wash, dry, concentrate and purify to obtain the pure product. The nitrile compound can be an aliphatic nitrile and an aromatic nitrile, and the substituent can be an alkyl group (C1-C12), an aromatic group with an electron-donating substituent or an electron-withdrawing substituent; the aliphatic nitrile includes acetonitrile, butyronitrile, heptane Nitrile, isopropionitrile, isobutyronitrile, etc.;
2. Used in the preparation process of one-dimensional single crystal titanium dioxide nanomaterials. One-dimensional nanomaterials have broad application prospects in the fields of optics, electronics, environment and medicine, and have become a hot topic in the field of materials research. As an n-type semiconductor and a typical photocatalyst, titanium dioxide is particularly attractive for its broad application prospects in photoelectric conversion, photovoltaic devices, photocatalytic degradation of pollutants, and photolysis of water to produce hydrogen. One-dimensional single crystal titanium dioxide nanomaterials are prepared by using titanium tetrachloride or titanium dioxide powder as raw materials and using a solvothermal method.
The method includes the following steps, and the amounts involved are in parts by weight:
1) Add 1 to 30 parts of titanium tetrachloride or titanium dioxide powder to 70 to 99 parts of alcohol, amine, nitrile or phenolic organic solvent, and stir vigorously to obtain uniformly dispersed titanium tetrachloride or titanium dioxide. A mixture of organic solvents;
2) Dissolve 10 to 60 parts of sodium hydroxide in 40 to 90 parts of deionized water, stir evenly, and obtain a transparent and clear sodium hydroxide aqueous solution;
3) Under rapid stirring, add 20 to 80 parts of the aqueous sodium hydroxide solution of step (2) to 10 to 50 parts of the mixture of step (1), continue stirring for a few minutes, and then transfer it to polytetrafluoroethylene. Lined in high pressure, at a constant temperature of 50~300℃;
4) Cool naturally, pour off the supernatant, wash with a dilute solution of deionized water and acid (concentration between 0 and 1.0M), centrifuge, collect the lower precipitate, and dry at 10 to 200°C to obtain a one-dimensional monolayer. Crystalline titanium dioxide nanomaterials. The constant temperature time of step (3) is 0.5 to 72 hours. The nitrile is an aliphatic nitrile or an aromatic nitrile, such as acetonitrile or o-methyl benzonitrile.
Main reference materials
[1] CN201510572882.3 A method for preparing high-purity o-methylbenzonitrile and its derivatives
[2] CN01141463.4 Methods for synthesizing multi-substituted pyridine derivatives and bipyridine derivatives from nitriles
[3] CN200410038073.6 Preparation method of one-dimensional single crystal titanium dioxide nanomaterials

 微信扫一扫打赏
微信扫一扫打赏

