Background and overview[1][2]
Photoinitiator (PI) is a key component of photocurable materials. It plays a decisive role in the photocuring speed of photocurable materials. Photoinitiator is a substance that can absorb radiant energy, undergo photochemical changes when excited, and produce active intermediates (free radicals or cations) with the ability to initiate polymerization. Photoinitiators can be divided into ultraviolet photoinitiators (absorbing ultraviolet light region 250-420nm) and visible light initiators (absorbing visible light region 400-700nm) due to differences in absorbed radiant energy.
Photoinitiators can be divided into two categories: free radical photoinitiators and cationic photoinitiators due to different active intermediates produced. Free radical photoinitiators can be divided into two categories: cracking photoinitiators and hydrogen abstraction photoinitiators due to different mechanisms of generating free radicals. At present, light curing technology is mainly ultraviolet light curing, and the photoinitiator used is ultraviolet light initiator. Because visible light initiators are sensitive to sunlight and general lighting sources, their production and use are limited, and they are only used in a few fields such as dentistry and printing and platemaking.
The research, development and production of photoinitiators in my country began in the 1970s. At that time, the main species were benzoin ether photoinitiators. Industrial production began in the mid-1990s and entered a stage of rapid development. Since 2000, my country’s photoinitiator production and export has ranked first in the world, becoming the world’s largest photoinitiator producer and exporter. With the development of light-curing technology, the application fields of light-curing products continue to expand, and the research and development and production of photoinitiators have also made great progress, mainly in the direction of low odor, low migration, good solubility, and high initiating efficiency. Therefore, there has been New photoinitiators such as macromolecular photoinitiators, macromolecular co-initiators, polymerizable photoinitiators, and free radical-cation hybrid photoinitiators have been developed.
Photoinitiator 369, chemical name is 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone, also known as photoinitiator IHT-PI 910. At present, there are two main process routes for synthesizing photoinitiator 369:
(1) This process uses chlorobenzene as raw material, and finally obtains 369 product through Friedel-Crafts reaction, bromination, dimethylamine substitution of bromine, quaternary ammonium salt synthesis, rearrangement, and morpholine substitution of chlorine. This process seems to have a low production cost, but the process of replacing chlorine with morpholine requires the use of dangerous high-temperature and high-pressure processes, with many by-products and low yields.
(2) This process uses fluorobenzene as raw material, and undergoes Friedel-Crafts reaction, bromination, dimethylamine substitution of bromine, morpholine substitution of fluorine, quaternary ammonium salt synthesis, and rearrangement to finally obtain the 369 product. This process uses relatively expensive fluorobenzene as raw material, has high selectivity and high yield during the reaction. Moreover, the morpholine substitution process does not require high temperature and high pressure, and has low equipment and operation requirements. However, due to the unsatisfactory yield of the quaternary ammonium salt step and the rising price of fluorobenzene, we had to seek a more cost-effective synthesis process route.
Apply[3][2]
Photoinitiator including photoinitiator 369 is one of the indispensable components of UV curing materials. In the photocuring system, including UV glue, UV coating, UV ink, etc., it undergoes chemical reactions after receiving or absorbing external energy. Change and decompose into free radicals or cations, thereby initiating polymerization reactions. Substances that can generate free radicals and further initiate polymerization upon exposure to light are collectively called photoinitiators. Radiation curing technology is a new energy-saving and environmentally friendly technology. It is cured by irradiation with ultraviolet (UV), electron beam (EB), infrared light, visible light, laser, chemical fluorescence and other radiation. It is fully in line with the “5E” characteristics: Efficient (high efficiency), Enabling (practical), Economical (economic), Energy Saving (energy saving), Environmental Friendly (environmentally friendly), so it is known as “green technology”.
Photoinitiator including photoinitiator 369 is one of the important components of photocurable adhesive, which plays a decisive role in the curing rate. After the photoinitiator is irradiated by ultraviolet light, it absorbs the energy of the light and splits into two active free radicals, triggering chain polymerization of the photosensitive resin and reactive diluent, causing the adhesive to cross-link and solidify. It is characterized by fast, environmentally friendly and energy-saving properties. It plays a decisive role in the sensitivity of the light-curing system. Since light-curing materials evaporate no solvent during curing, which greatly reduces environmental pollution, light-curing technology, as an environmentally friendly green technology, has developed vigorously in recent years. Examples of its application are as follows:
1. Preparation of a low-energy UV-curable card-making offset printing ink,
Includes the following mass percentage components: 40-50% bifunctional modified polyester acrylate; 5-10% dipentaerythritol penta/hexaacrylate; 5-ethoxylated trimethylolpropane trimethacrylate 10%; 1,6-hexanediol diacrylate 1-3%; photoinitiator combination 10-16%; pigment 15-20%; filler 3-5%; additive 1-3%. The photoinitiator combination includes the following mass percentage components: 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone (photoinitiator 369) 15-20%; phenyl bis( 2,4,6-trimethylbenzoyl)phosphine oxide 15-20%; 2,4,6-trimethylbenzoyl-diphenylphosphine oxide 15-20%; 2-dimethylamino-2 -Benzyl-1-[-4(4-morpholinyl)phenyl]-1-butanone 20-25%; 2,4-diethylthianthone 20-25%. The invention also discloses the preparation method and application of the ink.
2. Prepare an acid- and alkali-resistant UV glass edge sealing glue,
It mainly includes the following components: six-functionality PUA resin, weight ratio 30% to 50%; isobornyl acrylate, weight percentage 20%-40%; pentaerythritol triacrylate.�� Weight percentage 10%-25%; 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone (photoinitiator 369), weight percentage 1%-5%; Defoaming agent EFKA-2721, weight percentage 0.1%-5%; 3-(methacryloyloxy)propyltrimethoxysilane, weight percentage 0.1%-5%; red pigment, weight percentage 0.1%-1%; modification Polyacrylate, 1%-5% by weight.
Preparation[1]
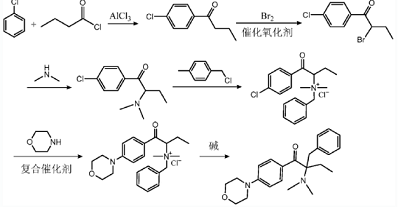
A synthesis method of 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone (photoinitiator 369), including:
(1) Under the catalysis of aluminum trichloride, chlorobenzene and n-butyryl chloride undergo Friedel-Crafts reaction to prepare compound I shown in formula (I);
(2) Under the action of catalytic oxidant, compound I and bromine undergo bromination reaction to prepare compound II represented by formula (II);
(3) Compound II undergoes a substitution reaction with dimethylamine to prepare compound III shown in formula (III);
(4) Compound III is quaternized with benzyl chloride to prepare compound IV shown in formula (IV);
(5) Under the action of a composite catalyst, compound IV and morpholine undergo a substitution reaction to prepare compound V represented by formula (V);
(6) Under the action of a base, compound V undergoes a rearrangement reaction to obtain the 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone (photo Initiator 369).
References
[1] CN201810101272.9 A synthesis method of 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)butanone
[2] CN201610355216.9 A kind of acid and alkali resistant UV glass edge sealing glue
[3] CN201810913988.9 A low-energy UV curing card-making offset printing ink and its preparation method and application

 微信扫一扫打赏
微信扫一扫打赏

