Background and overview[1]
Triphenylphosphine oxide, also known as triphenylphosphine oxide and triphenylphosphine oxide, is an organophosphorus compound. The oxyphenylphosphine oxymolecule has a tetrahedral structure, and the oxygen atom is basic. Due to the rigidity of the molecular skeleton and the basicity of the oxygen atoms, triphenylphosphine oxide can initiate crystallization of compounds that are difficult to crystallize by other methods. It is especially effective for acidic substances such as phenol.
Triphenyl phosphine oxide is commonly found in organic reaction by-products, such as Witting reaction, Staudinger reaction and Mitsunobu reaction, which all produce Ph3PO by-products, PPh3 Cl2 will also produce triphenylphosphine oxide when converting alcohols into chlorinated hydrocarbons. Ph3PO can coordinate with many “hard acid” metal atoms. Typical complexes such as tetrahedral NiCl2(OPPh3)2. Many metal ions can catalyze the oxidation reaction of PPh3 to Ph3PO, so triphenylphosphine samples often contain impurities triphenylphosphine oxide. Triphenylphosphine oxide is generally used as an organic synthesis intermediate, pharmaceutical intermediate, and can also be used as a catalyst and extraction agent.
Synthesis method[1]
Triphenylphosphine oxide is generally obtained by the oxidation reaction of triphenylphosphine.
2PPh3+O2→2Ph3PO
Application fields[2][3][4][5]
1. Organic synthesis
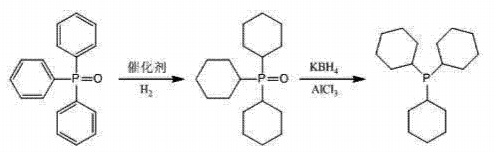
Ph3PO is an important raw material for organic synthesis and can be used to synthesize organic phosphine ligands such as tricyclohexylphosphine. The cyclohexyl group in tricyclohexylphosphine has good electronic effects and steric position. The hindrance effect makes the coordination effect better, and it is one of the important catalyst ligands in the coupling reaction. Using triphenylphosphine oxide as the starting material, catalytic hydrogenation under the action of the catalyst Ru/C, tricyclohexylphosphine oxide is synthesized, and then treated with KBH4-AlCl3Reduction synthesis of tricyclohexylphosphine. The reaction process is as follows.
2. Used as phase transfer catalyst
Use trimesic acid and thionyl chloride as raw materials, add the phase transfer catalyst Ph3PO, and react with reflux in the solvent benzene. After the reaction is completed, benzene and chlorine are removed by distillation under reduced pressure. sulfoxide, and then vacuum distillation to obtain trimesoyl chloride. The reaction yield reached 97.3%.
3. Preparation of new environmentally friendly flame retardants
Triphenylphosphorus oxide is a phosphorus oxide-based halogen-free flame retardant. It is mainly used for PC/ABS, EVA flame retardant, textile fabric flame retardant, polyethylene, polystyrene foam flame retardant, and is suitable for epoxy insulation. Material. Triphenylphosphine oxide can be compounded with other flame retardant materials to form a flame retardant with excellent flame retardant effect. It can also be added to flammable materials to perform flame retardant modification. For example, the flame retardant Ph3PO and bisphenol A-bis(diphenyl phosphate) are added to polyethylene terephthalate-1,4-cyclohexanedimethanol PETG. ) BDP, polyphenyl diphenyl sulfone phosphate PSPPP and appropriate nucleating agents and antioxidants are used to modify PETG. The resulting flame retardant material has excellent flame retardant effect, high light transmittance, and vertical combustion UL94 reaches V -Level 0, oxygen index 32%.
4. Used as catalyst ligand
Ph3PO is easy to coordinate with metals, and this property can be used to prepare organophosphorus coordination metal catalysts. Cobalt compounds such as cobalt acetate, cobalt chloride, etc. are used as catalyst precursors, and phosphorus oxides such as triphenylphosphine oxide are used as catalyst ligands to form a cobalt/phosphorus oxide catalyst system. Under mild conditions, the mixture is Isonononal is prepared from alkenes and synthesis gas (CO and H2).
Main reference materials
[1] Etter M C, Baures P W. Triphenylphosphine oxide as a crystallization aid[J]. Journal of the American Chemical Society, 1988, 110(2): 639-640.
[2] Yang Zhenqiang, Li Jiangtao, Wang Fuling, a method for producing tricyclohexylphosphine, CN 201210072957, application date 2012-03-20
[3] Xue Juqiang, Yang Deyao, Zhang Taiming, Preparation method of trimesoyl chloride, CN 201310647097, application date 2013-12-04
[4] Wu Jun, Zhang Jie, Zhang Zhaoju, a highly transparent halogen-free flame retardant material, CN 201610762250, application date 2016-08-30
[5] He Dehua, Wei Lan, Dong Guoli, method for producing isononyl aldehyde from mixed octene and synthesis gas, CN 200410062259, application date 2004-07-02

 微信扫一扫打赏
微信扫一扫打赏

