The synthesis of strong acid cation exchange resin is briefly introduced into two types: polymerization type and condensation type.
①Polymerization type: This method uses styrene and diethyl benzene to copolymerize into network polystyrene pellets, and then moraine preparation
Strong acid cation exchange resin is obtained.
②Condensation type: This method uses sulfate to decease phenol, so that the ring has an S (), H group, and then formaldehyde is used to react with it
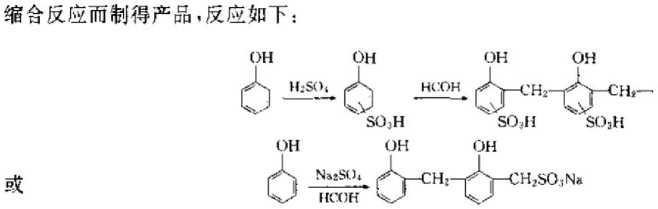
Synthesis of weakly acidic cation exchange resin
① The polymerization type uses acrylate monomer to initiate polymerization with divinylbenzene as a cross-linking agent, and then hydrolyzes it to obtain a weakly acidic ion exchange resin containing residues , the specific reactions are as follows;
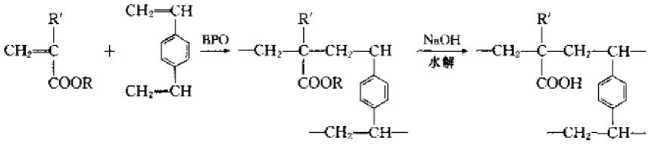
The reason why acrylate monomers are used in the reaction process is because if acrylic acid monomers are used for polymerization, acrylic acid is water-soluble and anode. Suspension polymerization cannot be carried out. into a ball. After using its esters to form balls through suspension polymerization, and then forming hydroxyl groups through hydrolysis reaction, a weakly acidic cation exchange resin can be obtained.
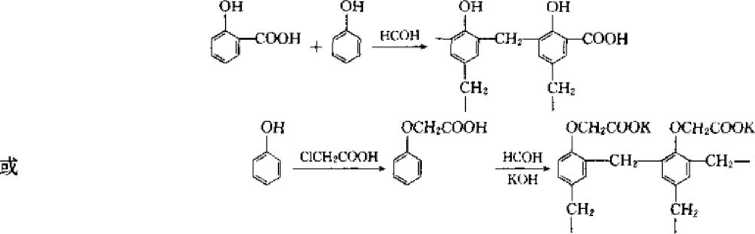
②Condensation type This is a synthesis method that obtains a weakly acidic cation exchange resin with a me group by condensing phenol with a hydroxyl group and formaldehyde. The chemical reaction is as follows:
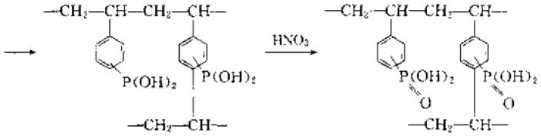
(3) Strongly basic ion exchange resin can control strong base ion exchange resin by adding strong basic organic amine groups to styrene and divinylbenzene copolymer beads. Basic ion exchange resin. The reaction is as follows:
(4) Weak base ion exchange resin If some weakly reactive groups are introduced into the copolymer beads used to synthesize strong ion exchange resin, That is, a weakly ion exchange resin can be obtained. The reaction formula is as follows;
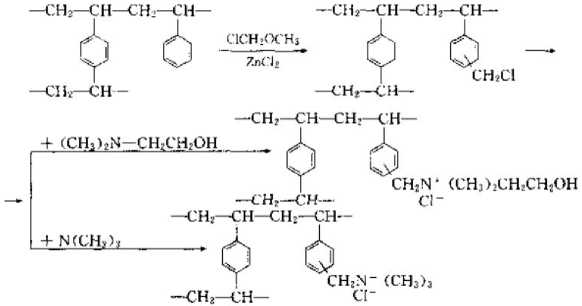
(5) Zwitterionic ion exchange resin is an ion exchange resin that has both acidic cation exchange groups and basic anion exchange groups.
The typical synthesis method is as follows:
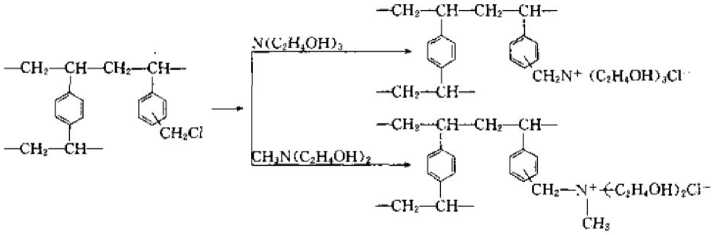
The most commonly used ion exchange resin in industrial production is pellets of about 1~2mm obtained by suspension polymerization of styrene-divinylbenzene. Ion exchange resins with different properties can be obtained after sulfonation, gas methylation and amination of the balls. At present, there are many brands of products produced in our country.

 微信扫一扫打赏
微信扫一扫打赏

