Background and overview[1]
2-Nitrophenyl-B-D-xylobioside can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
2-Nitrophenyl-β-D-xylobioside is prepared as follows:
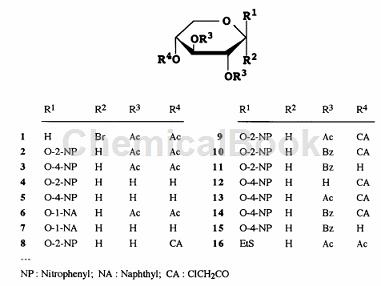
1) 1) Add anhydrous Me2CO (150mL) solution of 1 (41.2g, 0.12mol) dropwise to 2-nitrophenol (28.7g, 0.21mol) at room temperature within 30 minutes ) into a stirred solution of anhydrous Me2CO (700 mL). After stirring at room temperature overnight, the insoluble material was collected on a Celite pad, washed with MeCO, and the combined filtrate and washes were concentrated. A solution of the residue in 2:1 EtOAc-hexane (300 mL) was filtered through a layer of silica gel, which was washed with 2:1 EtOAc-hexane (500 mL). The combined filtrate and washes were concentrated, and the residue was crystallized from 2-PrOH-EtOH to give 2 (31.4 g, 65%):
2) A solution of 2 (29.6g) in anhydrous MeOH (300mL) was treated with a catalytic amount of methanol NaOMe. The mixture was kept at room temperature for 5 hours, neutralized with Amberlite IR-120 (H÷) resin, filtered and concentrated. The residue was crystallized from EtOH to give 4 (18.6 g, 92%): mp 172-174 °C; m/z.
3) Boil and reflux a suspension of 4 (4.13g, 15.2mmol) and dibutyltin oxide (4.17g, 16.7mmol) in MeOH (120mL). After approximately 30 minutes, the mixture became homogeneous and heating was continued for 1.5 hours, during which time the solution was concentrated to half of its original volume using a Dean-Stark condenser. The mixture was cooled to room temperature and concentrated to dryness. To a stirred solution of the residue in anhydrous CH2Cl2 (70 mL) was added dropwise a solution of CICHECOC1 (1.33 mL, 16.7 mmol) in CH2Cl2 (15 mL) at 0 °C, and the mixture was stirred at 70 °C for 30 min. 0°C and then concentrated. The residue was purified by column chromatography (2:1 PhMe-EtOAc) to give 8 (3.71 g, 70%).
4) To a stirred solution of 8 (5.90g, 17mmol) in CH2Cl2 (90mL) containing pyridine (11.0mL, 0.14mol) at -5°C, BzCl (7.88mL) was added dropwise , 68mmol) solution of CH2Cl2 (7.85mL, 68mmol) 20mL. The mixture was stirred at 0°C for 5 hours and worked up as above. The residue was purified by column chromatography (60:1 PhMe-EtOAc) to afford amorphous 10 (8.58 g, 91%).
5) Boil a mixture of 10 (7.89g, 14.2mmol) and (NH2)2C=S (5.40g, 70.9mmol) in MeOH (100mL) under reflux for 2h. The mixture was concentrated and the residue was partitioned between CH2Cl2 and aqueous NaHCO3. The organic layer was washed with H2O, dried and concentrated. The residue was purified by column chromatography (6:1PhMe-EtOAc) to obtain amorphous 11 (6.12g, 90%):
6) At 0°C, to a stirred mixture of 11 (1.76g, o3.7mmol), 16 (1.53g, 4.8mmol) and powdered 4A molecular sieve, add CH2Cl2 (30mL ), add NIS (1.06g, 4.8mmol)), and then add dropwise a solution of silver triflate (0.19g, 739/zmol) in PhMe (5mL). After 10 minutes, filter the mixture through a diatomaceous earth layer into ice water and distribute the filtrate. The organic layer was washed successively with Na2S2O3 aqueous solution, NaHCO3 aqueous solution and H2O, dried and concentrated. Column chromatography of the product (10:1PhMe-EtOAc) gave 2-nitrophenyl O-(2,3,4-tri-O-acetyl-fl-D-xylopyranosyl)-(1~4 )-2,3-di-O-benzoyl-fl-D-pyranopyranoside (17) (2.17 g, 80%): mp 175-176°C.
7) A solution of 17 (1.02g) in MeOH (20mL) and CH2Cl2 (5mL) was treated with methanol NaOMe (0.5mL) to give 29(2-nitrophenyl-β- D-xylobioside) (0.85g, 83%): mp95-97.5°C
Main reference materials
[1] Synthesis of 2- and 4-nitrophenyl/3-glycosides of 13-(1 —> 4)-D-xylo-oligosaccharides

 微信扫一扫打赏
微信扫一扫打赏

