Overview
Boc-D-Homophenylalanine is an amino acid raw material that can be used to prepare peptides.
Preparation method[1-2]
Method 1. Chacko et al. reported that Boc-D-homophenylalanine can be synthesized through the following route.
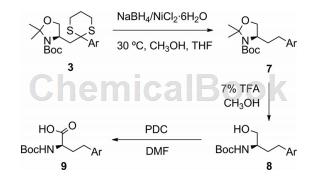
Method 2,
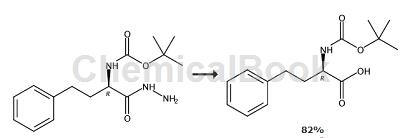
Preparation of (2R)-N-amino-2-[(tert-butoxy)carbonylamino]-4-phenylbutanamide: HD-Hphe-OH (44.0g, 245mmol) in 1:1 H2O/t-BuOH (350 mL) was treated with powdered NaOH (10.8 g, 270 mmol) at 22°C followed by three aliquots of Boc2O (58.9g, 270mmol). The resulting suspension was stirred for 16 hours, then diluted with H2O (300 mL) and washed with Et2O (3 × 200 mL). The remaining aqueous solution was acidified (pH 5) with AcOH and washed with ethyl acetate (3 × 250 mL). NOTE: During the ethyl acetate wash, add additional AcOH to the aqueous layer to maintain pH. The combined organic extracts were washed with H2O (250 mL) and saturated NaCl (250 mL), then dried over Na2SO4, Filtration and concentration in vacuo gave a white solid (47.7 g, 171 mmol). The original Et2O wash solution was acidified with aqueous AcOH solution to precipitate unreacted amino acids (6.8 g, 38 mmol). Additional Boc-protected material (8.51 g, 30.4 mmol) was recovered from the filtrate using the extraction procedure described above; combined yield, 56.2 g, 201 mmol, 82.0%. The product was used in subsequent reactions without further purification.
Main reference materials
[1] Synthesis of γ-oxo γ-aryl and γ-aryl α-amino acids from aromatic aldehydes and serine ,By Chacko, Shibin and Ramapanicker, Ramesh.
From European Journal of Organic Chemistry, 2012(36), 7120-7128; 2012
[2] PCT Int. Appl., 2007005491, 11 Jan 2007

 微信扫一扫打赏
微信扫一扫打赏

