Overview[1]
Methyl 4-hydroxyphenylacetate is an organic intermediate that can be used to synthesize 4-isobutoxybenzylamine. 4-isobutoxybenzylamine is a key intermediate in the synthesis of pimaserin and has broad applications. market prospects.
Apply[1]
Methyl 4-hydroxyphenylacetate can be used to synthesize 4-isobutoxybenzylamine.
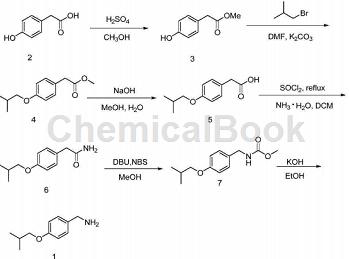
Using 4-hydroxyphenylacetic acid (2) as raw material, esterification reaction produces 4-hydroxyphenylacetic acid methyl ester (3); (3) substitution reaction produces 4-isobutoxyphenylacetic acid methyl ester (4) ), the obtained product (4) is hydrolyzed to obtain 4-isobutoxyphenylacetic acid (5); (5) undergoes acylation reaction to produce 4-isobutoxyphenylacetamide (6); (6) is reacted by Hofmann Furman) degradation reaction generates N-(4-hydroxybenzyl) methyl carbamate (7); (7) is hydrolyzed to obtain the target product (1).
Preparation[1]
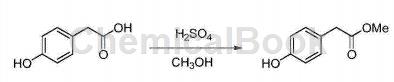
Synthesis of methyl 4-hydroxyphenylacetate:
Add p-hydroxyphenylacetic acid (5.0g (gram), 32.86mmol (millimol)) into a 100mL (ml) round-bottomed flask, then add 30mL methanol to dissolve it, and add dropwise 2.5mL of 98% concentrated sulfuric acid. , heated to 80°C (Celsius) for reflux reaction, and after 0.5h (hour) it was detected by TLC (thin layer chromatography) and the reaction was completed. Spin off the methanol under reduced pressure at 50°C, add 100 mL of water, extract with ethyl acetate (50 mL × 3), combine the organic phases, wash with saturated brine (100 mL × 3), and finally dry with anhydrous sodium sulfate, spin dry to obtain yellow color Oily substance 4-hydroxyphenylacetic acid methyl ester 3 5.3g, yield 91.58%. 1H NMR (400MHz, CDCl3) δ: 7.08 (d, J=8.3Hz, 2H), 6.73 (d, J=8.3Hz, 2H), 3.69 (s,3H),3.55(s,2H);13C NMR (100MHz, CDCl3)δ: 40.30, 51.97, 115.96, 125.40, 130.40, 156.16, 172.52.
Main reference materials
[1] [Chinese invention] CN201810213656.X A synthesis method of 4-isobutoxybenzylamine

 微信扫一扫打赏
微信扫一扫打赏

