Background and overview[1]
Benzofuran and its derivatives such as 5-bromobenzofuran are very important organic compounds with high biological activity and are widely present in the skeleton of many natural products. Therefore, the importance of benzofuran and its derivatives Synthesis has received widespread attention. Traditional methods play an important role in the synthesis of bioactive benzofurans, but there are common problems such as too long routes, harsh substrate and reaction conditions, and limited expansion of substituted functional groups. Using palladium and other precious metals as catalysts to synthesize benzofuran can overcome the shortcomings of traditional synthesis methods and further improve the yield, but the cost is higher. In recent years, cheap transition metal copper has gradually become a new focus of research. Research on using copper as a catalyst to synthesize benzofurans has achieved promising results, but there are still limitations in the substrates and the relatively expensive reagents used. shortcoming. Although these results are very successful, their industrial production has encountered great challenges, and the heavy metal catalysts used will cause environmental pollution.
Preparation[1]
5-Bromobenzofuran is prepared as follows:
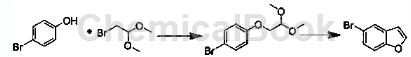
Add 1,4-dioxane (800mL) into a 2000mL three-neck round bottom flask, then add p-bromophenol (210g, 1.213mol), potassium carbonate (750g, 5.426mol) and 2-bromophenol in sequence Acetaldehyde dimethyl acetal (300g, 1.775mol). Start stirring and heat to reflux for 24 hours. Reactions were followed by TLC and GC. After the reaction is completed, most of the 1,4-dioxane is distilled off. Add 600 mL each of water and ethyl acetate to the residue, stir the mixture for 30 minutes, and separate the organic phase. The aqueous phase was extracted with 200 mL of ethyl acetate, and the organic phases were combined and dried over sodium sulfate overnight. The obtained crude product (340g) was directly used in the next reaction. Add chlorobenzene (1200mL) to a 5000mL three-neck round bottom flask, then add the above crude product 1-bromo-4′-(2,2-dimethoxyethyl)benzene (340g) and phosphoric acid (850g) in sequence. . Start stirring and heat to reflux for 24 hours. Reactions were followed by TLC and GC. After the reaction is completed, the reaction solution is cooled to room temperature, and the lower layer is separated. The organic phase was washed with water (600 mL) and sodium hydroxide (2 mol/L, 500 mL). Dry over sodium sulfate overnight. Most of the chlorobenzene is removed by distillation. Then it was changed to distillation under reduced pressure to obtain crude product 5-bromobenzofuran (205g). After distillation, 149g of pure 5-bromobenzofuran was obtained with a yield of 62.3%.
Main reference materials
[1] (CN103724304) Preparation method of 5-bromobenzofuran

 微信扫一扫打赏
微信扫一扫打赏

