Overview[1][2]
3-Phenylbut-2-enoic acid can be prepared in one step from triethyl phosphonoacetate and phenylacetaldehyde, and can be used to synthesize 2-position disubstituted indolin-3-one compounds. Substituted indolin-3-one structures are widely found in biologically active natural product molecules.
Preparation[1]
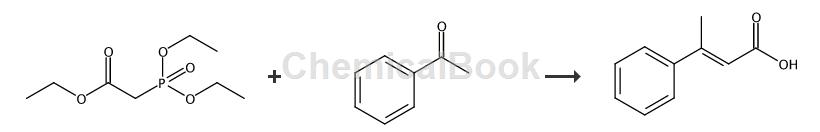
In a 100 mL two-neck round-bottom flask equipped with a magnetic stir bar, thermometer and condenser, add triethyl phosphonoacetate (1.344 g, 6 mmol), then add THF (20 mL). Cool the flask to 5 °C and add NaH (60% in mineral oil, 265 mg, 6.6 mmol) portionwise over 10 min. The flask was warmed to approximately 25°C and phenylacetaldehyde (6 mmol) was added to the clear solution via the addition funnel. Heat the flask to reflux for 8 hours, then carefully quench the reaction by slowly adding H2O (warning: vigorous gas evolution). The contents of the flask were then poured into water and extracted with ether and washed with 0.1N HCl and brine. The solvent was removed and a crude oil was obtained. The resulting oil, THF (2 mL) and 2 M NaOH (2.4 mL, 4.8 mmol) were charged into a 50 mL single-neck round-bottom flask, and heated to reflux for 3 h. Dilute the contents with Et2O and acidify with concentrated hydrochloric acid. Extract twice with Et2O, wash with brine, dry over MgSO4, filter and concentrate to obtain the product, 1 g of α,β-unsaturated carboxylic acid.
Apply[2]
CN201910513544.0 reports the synthesis of 2-position disubstituted indolin-3-one compounds using 3-phenylbut-2-enoic acid.
Place (E)-3-phenylbut-2-enoic acid 35.1mg (0.24mmol), 2-phenyl-3H-indol-3-one 41.4mg (0.2mmol), and carbene catalyst 7.00mg ( 0.02mmol), 1H-benzotriazole-1-yloxytripyrrolidinyl hexafluorophosphate 156mg (0.72mmol), cesium carbonate 195.4mmol (0.6mmol) and 4mL of diethyl ether were placed in a 25mL two-neck bottle at room temperature. The reaction solution was reacted for 4 hours. The reaction solution was cooled and concentrated. It was eluted by column chromatography using a mixed solvent with a petroleum ether: acetone ratio of 20:1 as the eluent. The eluate portion of all the products detected was collected and the solvent was evaporated. Finally, 64 mg of product was obtained, with a yield of 90%.
Main reference materials
[1] From Synthesis, 46(5), 607-612, 6 pp.; 2014
[2] [Chinese invention] CN201910513544.0 A 2-position disubstituted indoline-3-one compound and its asymmetric synthesis method

 微信扫一扫打赏
微信扫一扫打赏

