Background and overview[1][2]
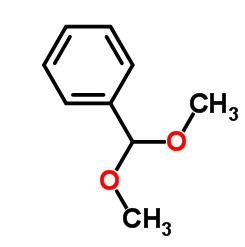
Benzaldehyde dimethyl acetal is also known as dimethoxybenzylmethane. Colorless liquid, with a soft, sweet and rich green aroma similar to benzaldehyde. Relative density 1.0254, boiling point 198℃ (199℃). It is more stable than benzaldehyde in alkaline media and is not easily oxidized; it is easily decomposed into benzaldehyde and methanol under acidic conditions. It can be obtained by the dehydration condensation reaction of benzaldehyde and methanol. It is used for spices. It can be used as the green-floral top fragrance component of the lily of the valley or narcissus fragrance base, which can have unique effects.
Benzaldehyde dimethyl acetal is a liquid with a similar almond aroma and is found in potato seeds. It is easy to hydrolyze to generate benzaldehyde under acidic conditions, but it is not affected by alkaline environment. It is an important form of preserving benzaldehyde at the temperature of oxidizing agent or reducing agent. Benzaldehyde dimethyl acetal can be used as a raw material for food flavors and daily chemical essences, and can also be used as a reagent for the synthesis of selenium carbonyl compounds.
Preparation[3]
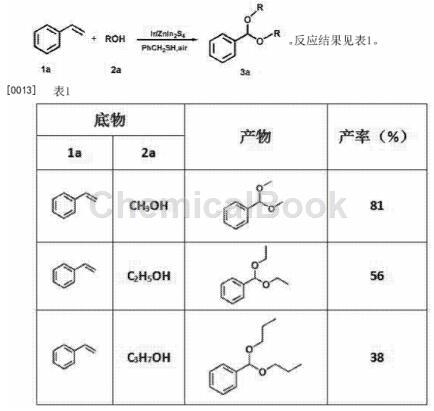
CN201811481556 reports a method for synthesizing aromatic acetals from styrene under visible light. 8-10mg of the catalyst Ir/ZnIn2S4 is added to the reaction tube, and a certain proportion is added of styrene and mercaptan (the molar ratio of styrene to mercaptan is 1.2:1-1:1) and 4 ml of alcohol, and then irradiate the reaction tube under normal temperature, normal pressure, air atmosphere, and visible light for 8-15 hours. Thiols generate thiol radicals under the action of catalyst Ir/ZnIn2S4 and visible light. The generated thiol radicals react with styrene to generate alkyl radicals, and generate peroxy radicals under the action of O2 in the air, and then generate Peroxyl radicals react with alcohol to eventually form acetal. The method does not require the addition of protonic acid or Lewis acid, and the reaction time is 8-15h. The reaction formula is as follows:
Main reference materials
[1]Dictionary of Chemical Substances
[2][China Invention] CN201110385999.2 Supported catalyst for the synthesis of benzaldehyde dimethyl acetal and its preparation method and application
[3][Invented in China] CN201811481556.1 A method for synthesizing aromatic acetals from styrene under visible light

 微信扫一扫打赏
微信扫一扫打赏

