Background and overview[1-4]
2-Nitro-1,4-phenylenediamine, also known as 2-nitro-1,4-phenylenediamine, is an important pharmaceutical raw material that can be used to synthesize retigabine, ilaprazole, and izoga Bin and other drugs.

Preparation[1]
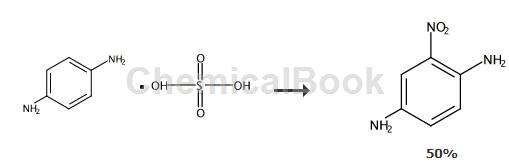
Place 0.5 mol (approximately 103.1 g) of 1,4-p-phenylenediamine sulfate I, 1.1 mol (approximately 231 g) of analytically pure trifluoroacetic anhydride, 1.6 mol (approximately 162 g) of triethylamine and 250 ml of analytically pure dichloromethane was added to the reactor and stirred thoroughly at room temperature for 1 hour. After filtration, 142.5 g of N1, N4-ditrifluoroacetyl-p-phenylenediamine II was obtained.
Add N1, N4-ditrifluoroacetyl-p-phenylenediamine II (60.3 g, 0.2 mol) to 300 ml of analytically pure acetic anhydride, and stir thoroughly to form a suspension. Add 25 ml of analytical grade concentrated nitric acid dropwise to the reaction system at room temperature. After the addition is completed, stirring is continued for 6 hours. The reaction solution was then filtered, and the filter cake was washed once with 200 ml of water and 200 ml of analytically pure ethyl acetate. After sufficient drying, 36.3 g of N1, N4-ditrifluoroacetyl-2-nitro-p-phenylenediamine III was obtained.
Add 36.3 grams of N1, N4-ditrifluoroacetyl-2-nitro-p-phenylenediamine III, 53 grams of sodium carbonate and 400 ml of water into the reactor, and heat to reflux for 1 hour. After the reaction was completed, the reaction system was lowered to room temperature. The reaction system was suction filtered to obtain 16.1 g of 2-nitro-p-phenylenediamine. The total yield of the three-step reaction is 50%; the product is a red-black solid with a melting point of 136-137°C. Hydrogen nuclear magnetic resonance spectrum: (1HNMR, 300MHz, d-DMSO) δ7.45 (s, 1H), 7.07 (s, 1H), 6.90 (s, 2H), 5.03 (s, 2H) ); Mass spectrometry (EI): 153(100), 107(99).
Apply[2-4]
CN201310309028.9 provides a method for the preparation of retigabine intermediates. Retigabine is a synthetic aminopyridine analogue that has activities such as anticonvulsant, antipyretic and analgesic, and can block sodium and calcium Current, enhance the current induced by GABA in neuronal cells, etc. The method includes the following steps: p-fluorobenzaldehyde reacts with 2-nitro-p-phenylenediamine in an alcohol solvent under the action of a reducing agent to obtain 2-amino-5-[(4-fluorobenzyl)amino]-1 -Nitrobenzene, the reducing agent is sodium triacetoxyborohydride or sodium cyanoborohydride. The invention also provides a preparation method of retigabine. The method does not require the use of toxic solvents and expensive catalysts, is economical, environmentally friendly, low in cost, and does not require complex post-processing. At the same time, the present invention uses sodium triacetoxyborohydride or sodium cyanoborohydride as the reducing agent, and the one-pot reaction is relatively complete, which greatly improves the yield of the target product. Experiments show that the yield of the preparation method provided by the invention can reach more than 90%.
CN201310702091.9 provides a method for preparing ilaprazole. Ilaprazole is a new generation of proton pump inhibitor (PPI), and its chemical structure belongs to benzimidazole derivatives. The drug was developed by South Korea’s Ilyo Pharmaceutical Co., Ltd. and was first launched by Livzon Group. This method uses 2-nitro-p-phenylenediamine as the starting material to prepare the important intermediate 5-(1H-pyrrole-1-yl)-2-mercaptobenzimidazole through the above route, and then docking and oxidation to obtain ilaprazole , the present invention not only provides a new synthesis route, but also provides a new method for purifying ilaprazole. This synthesis route has a high yield, is simple to operate, does not require special equipment, and is easy for industrial production. The purification method is simple to operate, and the product is obtained in one process without repeated purification, saving a lot of manpower, material resources and time, and the product has high purity and good quality.
CN201310131178.5 discloses a synthesis method of 4-(4-fluorobenzylamino)-1,2-phenylenediamine, the intermediate of ezagabine. Ezogabine, chemical name: N-[2-amino-4-(4-fluorobenzylamino)phenyl]ethylcarbamate dihydrochloride, trade name: Potiga, is produced by Valeant Pharmaceuticals and A new anti-epileptic drug jointly developed by GlaxoSmithKline (GSK). The drug is a neuronal potassium channel opener. It was launched in Europe in March 2011. It is called retigabine and its trade name is Trobalt. In June 2011, it was approved by the US FDA for the auxiliary treatment of partial epileptic seizures in adults. The invention uses 2-nitro-p-phenylenediamine and p-fluorobenzaldehyde as raw materials, and obtains the intermediate 4-(4-fluorobenzylamino)-2- through a one-pot two-step reaction of nucleophilic addition/dehydration and reduction. Nitroaniline (Formula B). Synthesis of Izogabine intermediate 4-(4-fluorobenzylamino)-1,2-phenylenediamine by reduction of 4-(4-fluorobenzylamino)-2-nitroaniline using ammonium sulfate-sodium borohydride system . The reaction has the advantages of short reaction time, simple post-treatment, and high yield.
Main reference materials
[1]FromFamingZhuanliShenqing,101935284,05Jan2011
[2]CN201310309028.9 Preparation method of retigabine intermediate and preparation method of retigabine
[3]CN201310702091.9 Preparation method of ilaprazole
[4]CN201310131178.5 A method for synthesizing ezagabine intermediates

 微信扫一扫打赏
微信扫一扫打赏

