Background and overview[1]
M-fluorobenzaldehyde is an important organic synthesis intermediate. It is a colorless or light yellow liquid with a pungent odor and can be dissolved in many organic solvents such as ethanol, ether, methylene chloride, toluene, etc. Boiling point 66-68℃, flash point 56℃, relative density 1.17. m-Fluorobenzaldehyde is easily oxidized to m-fluorobenzoic acid by oxygen in the air, so it should be stored in a sealed container. m-Fluorobenzaldehyde can be widely used in the synthesis of fine chemicals such as medicines, pesticides, and plastic additives.

Preparation[1]
A method for continuously oxidizing m-fluorotoluene to prepare m-fluorobenzaldehyde using a tubular reactor with a special structure, which is carried out according to the following steps:
(1) First, at room temperature, stir and mix the substrate m-fluorotoluene and part of the carboxylic acid solvent in a volume ratio of 1:1, mix the oxidant and part of the carboxylic acid solvent in a volume ratio of 1:1, and then The metal complex is mixed and poured into the m-fluorotoluene-carboxylic acid solution, and the sodium salt is poured into the hydrogen peroxide-carboxylic acid solution; through the required reaction time, the different flow rates of the two materials are calculated, and the different flow rates of the two materials are continuously pumped through the metering pump. It enters the tubular reactor and is preheated and mixed before entering the reaction zone for reaction. The reaction temperature is controlled by an external circulation heat exchange system;
(2) Control the molar ratio of the reaction materials by adjusting the flow rate and weighting, and control the residence time of the material mixing reaction from 60 to 1800s by changing the inner diameter of the pipe of the tubular reactor from 0.5 to 15 mm and the volume from 25 to 750 ml; After the reaction is completed, the product flows out from the end of the reactor into the collection tank. The product is distilled and separated. The unreacted m-fluorobenzaldehyde is circulated and reacted. The product m-fluorobenzaldehyde is collected after distillation and purification. The yield of the target product m-fluorobenzaldehyde is It can reach 20%~60%.
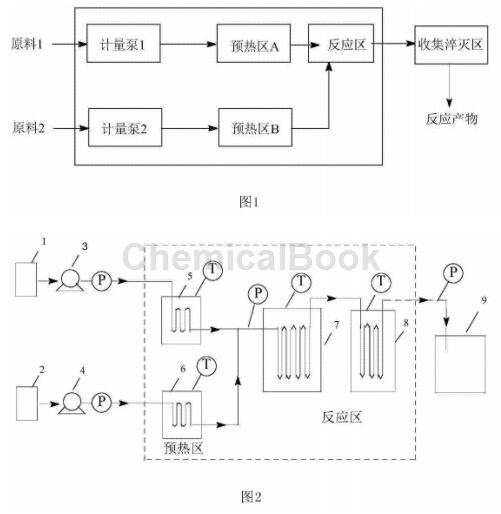
Fig. 1 is a process flow chart for preparing m-fluorobenzaldehyde by continuous oxidation of m-fluorotoluene according to the present invention.
Figure 2 is a diagram of the continuous flow tubular reactor device used in the present invention: 1, 2-raw material tank, 3, 4-raw material metering pump, 5-preheating zone, 6, 7-reaction zone, 8- Product quench collection area.
Figure 3 is a schematic diagram of the channel structure of the tubular reactor used in the present invention, in which a – direct flow channel structure, b – round cake type pulse reducing type rectangular flat pipe, c – rhombus pie type pulse reducing type Rectangular flat pipe, d-enhanced hybrid round cake flat pipe, e-Corning’s HeartCell structure microchannel.
Apply[2-3]
Metafluorocinnamic acid is an organic synthesis intermediate and chemical reagent. It is mostly used in organic synthesis, development of fine chemicals and drug research and development. CN201610049436.9 discloses a synthesis method of m-fluorocinnamic acid, which includes the following steps: add m-fluorobenzaldehyde and pyridine to the reaction vessel, stir and heat to 90°C~110°C, add malonic acid in batches under stirring until it is completely dissolved , after the addition is completed, stir and reflux at 90°C to 110°C for 1.5 to 2.5 hours; add dilute hydrochloric acid to the reaction vessel, cool to room temperature, and filter with suction to obtain a solid. Wash the solid with hot water until the pH of the wash liquid is neutral and colorless. Dry to get the finished product. The beneficial effects of the present invention are: reducing the amount of solvent and catalyst, shortening the synthesis reaction time, optimizing the post-treatment process, and improving product purity; the purity of the crude product is 99.83%, and the purity after recrystallization reaches 99.95%.
1-Fluoro-3-[2-(trans-4-alkylcyclohexyl)ethyl]benzene is an important liquid crystal intermediate and is often used to prepare liquid crystal compounds with excellent properties. CN201410168832.4 discloses a synthesis method of 1-fluoro-3-[2-(trans-4-alkylcyclohexyl)ethyl]benzene, using m-fluorobenzaldehyde as raw material, and 1-(trans- The Grignard reagent prepared from 4-alkylcyclohexyl)-2-bromomethane undergoes Grignard reaction, and after dehydration, 1-fluoro-3-[2-(trans-4-alkylcyclohexyl)vinyl]benzene is obtained, and then Catalytic hydrogenation yields 1-fluoro-3-[2-(trans-4-alkylcyclohexyl)ethyl]benzene. The method has simple operation, few reaction steps, mild reaction conditions, no pollution, low cost of raw materials used, and the prepared 1-fluoro-3-[2-(trans-4-alkylcyclohexyl)ethyl]benzene has high purity, The total yield can reach 63%~70%, which is suitable for industrial production.
Main reference materials
[1]CN201610972046.9 A method for preparing m-fluorobenzaldehyde by continuous oxidation of m-fluorotoluene
[2][China invention, China invention authorization] CN201610049436.9 Synthesis method of m-fluorocinnamic acid
[3]CN201410168832.4 A synthesis method of 1-fluoro-3-[2-(trans-4-alkylcyclohexyl)ethyl]benzene

 微信扫一扫打赏
微信扫一扫打赏

