Background and overview[1]
3-Fluoro-4-nitrotoluene can be used as a pharmaceutical synthesis intermediate. It can be obtained by one-step nitration of 3-fluorotoluene.
Preparation[1]
3-Fluoro-4-nitrotoluene is prepared as follows:
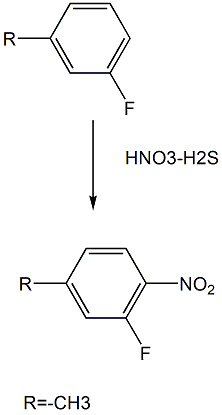
The specific steps are as follows: While stirring 160cm3 of concentrated nitric acid on an ice bath, add 160cm3 of concentrated sulfuric acid dropwise below 10°C. Add 90g of 3-alkylfluorobenzene dropwise to the solution while stirring on an ice bath at 5-10°C, then stir on an ice bath for 1 hour and at room temperature for 1 hour. The reaction solution was poured into 500g of ice, extracted with chloroform, and washed with water. The residue was recrystallized from hydrated methanol to obtain 100 g of 3-fluoro-4-nitrotoluene.
Application[1]
3-Fluoro-4-nitrotoluene can be used as a pharmaceutical synthesis intermediate. If it can be used to prepare 2-fluoro-4-methylbromobenzene: Dissolve 100g of 2-fluoro-4-methylnitrobenzene in 500cm3 of ethanol, add 1.6g of 5% palladium on carbon, and stir vigorously absorb hydrogen gas. The reaction product was filtered to remove the palladium on carbon, and the ethanol in the filtrate was distilled off. The remaining oil was distilled under reduced pressure (boiling point 71°C/10mmHg) to obtain 73.5g of 2-fluoro-4-methylaniline. 73.5 g of 2-fluoro-4-methylaniline were dissolved in a solution obtained by diluting 280 cm3 of 48% hydrobromic acid with 280 cm3 of water. On an ice bath, add a solution prepared by dissolving 42 g of NaNO2 in 90 cm3 of water at 5°C or lower. The diazonium salt solution was added dropwise to a solution obtained by dissolving 100 g of CuBr in 48% hydrobromic acid with stirring, and then stirred at room temperature overnight. The reaction solution was extracted with chloroform, and washed with 48% hydrobromic acid and water. Chloroform was distilled off, and the remaining oil was distilled under reduced pressure (boiling point 68°C/10mmHg) to obtain 94 g of 2-fluoro-4-methylbromobenzene.
Main reference materials
[1]JP1993117206 – Ethyla compounds and びそれをcontaining する liquid crystal compositions and びその liquid crystal compositions are represented by いた liquid crystals

 微信扫一扫打赏
微信扫一扫打赏

